Chemistry Reference
In-Depth Information
inhibitors (i.e. scaffold with annealed amine reagent conformer) were energy minimized
in the BACE-1 active site using the Merck Molecular Force Field (MMFF).
[
36
]
The best
conformer/pose for each reagent was selected using an energy-based scoring metric
[
37
]
that
emphasizes poses which combine a low conformational energy with a favorable enzyme-
inhibitor interaction energy. An arbitrary interaction energy cut-off of -45 kcal mol
−
1
was
employed to reduce the number of amines for consideration. Even so, more than 500 amine
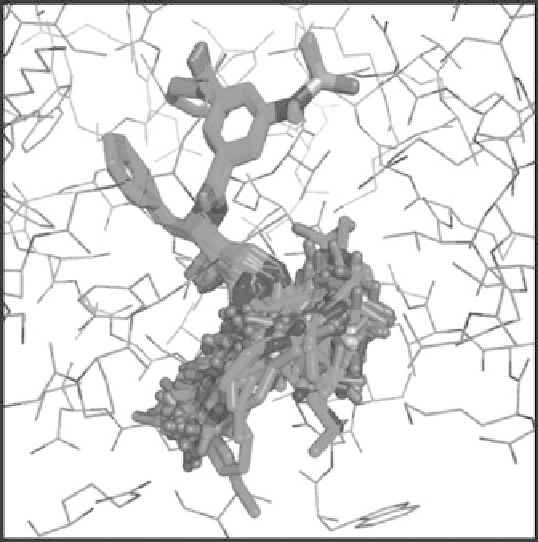
reagents were selected. The active site space sampled by the amine reagents is illustrated
in Figure 9.11.
Figure 9.11
Sampling the S1
pocket by conformational search over amine fragments.
Based on these results, it was hypothesized that the S1
pocket of BACE-1 is rather large,
open and promiscuous. To examine this hypothesis, a test set of BACE-1 inhibitors was
employed that included (a) previously synthesized compounds containing this scaffold
and a variety of P1
amine reagents, (b) new compounds with amine reagents selected
independent of the virtual reagent scan and scoring metric and (c) new compounds that
contained amine reagents which scored well in the virtual reagent scan. After synthesis and
assay of (b) and (c) inhibitors, a plot of observed activity versus energy score was obtained,
as shown in Figure 9.12.
It is clear that both the virtual reagent scan and the independent selection of reagents by
the chemist led to active BACE-1 inhibitors. Thus, virtual selection of reagents succeeded
in raising interesting reagents to the top of the list, although in some cases their activity
appears to have been under-predicted (see the two ether-containing amines highlighted in