Chemistry Reference
In-Depth Information
et al
.'s work highlights the ability of virtual screening to identify fragments which bind in
the BACE-1 active site, albeit with low computed LE and BEI values. Although scoring
functions are not ideal, they are still able to rank molecules with reasonable reliability.
The work of Murray
et al
. and others lends support to the concept of combining virtual
screening of fragments prior to analysis of binding by X-ray crystallography.
N
H
H
N
+
N
Asp32
H
H
Asp228
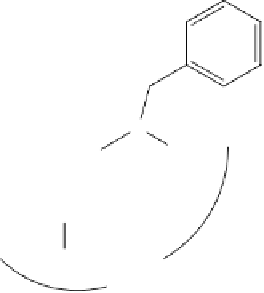
Figure 9.9
310
μ
M BACE-1 compound obtained by Murray
et al
.
[23]
through virtual
screening.
Polgar and Keserü have demonstrated that the incorporation of pharmacophoric con-
straints can increase the rate of retrieving actives from a 3D virtual screen by limiting the
possible conformations and docking poses to the most interesting ones.
[
30
]
Using BACE-
1 as the target and optimizing the protonation states of the catalytic aspartic acids, they
included pharmacophoric constraints derived from a Vertex patent
[
31
]
and were further able
to improve their enrichment factor from 36 to 41.
[
30
]
In another report, Hajduk and Greer described the successful application of structure-
based design in the drug discovery process.
[
32
]
Their work might lead one to believe
that having the structure of the target protein
should
increase one's probability of suc-
cess; however, in practice, computational chemists do not find that to be true.
[
33
]
In this
recent virtual screening study, the authors contend that if one is solely measuring suc-
cess by the number of active molecules found (or 'hits'), then a simple 2D methodology
is best.
9.5 Elaboration of a Fragment via Synthesis
Baxter
et al
. have nicely demonstrated that one can take a 0.9 Mhit, discovered fromHTS,
to an 11 nM
K
i
compound.
[
34
]
An X-ray crystal structure of the HTS lead helped demonstrate
the unique binding pose in the active sitewhich only occupied the non prime side of BACE-1
in a hairpin orientation. The crystal structure revealed that the prime side was not occupied
and the incorporation of a cyclohexyl ring resulted in a potent 11 nM compound.
[
33
]