Chemistry Reference
In-Depth Information
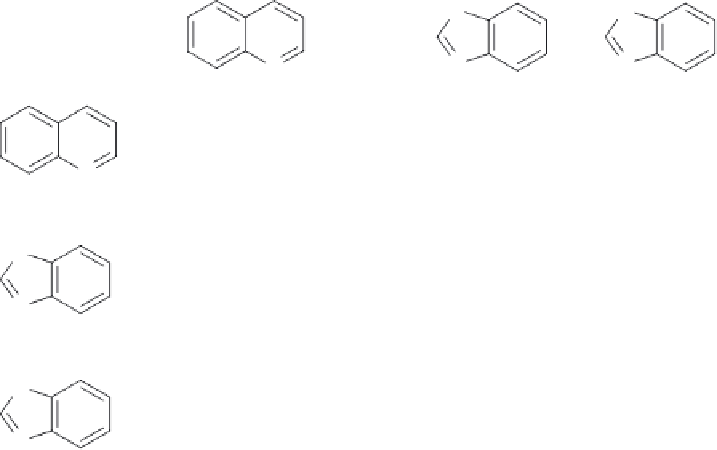
Table 8.3
Pairwise comparison of bicyclic rings (taken from Kho
et al
.
[37]
).
a
S
O
.
.
.
N
N
N
↑
↑
-3.0331
0.000
.
.
.
-1.0182
N
…
.
.
.
.
.
.
S
↑
0.000
N
-2.0149
Benzothiazole
O
0.000
N
Benzoxazole
a
The numbers are the logarithm of the odds ratio and indicate the preference in terms of mutagenic potential of one
ring system relative to the other. For instance, a value of -1.0182 (second row, second column from the right) means
that the left ring system has higher odds of being found in Ames positive compounds, so the top ring system is preferred.
The arrow points to the fragment that is more likely to be found in the Ames-negative class. Many more ring systems
were considered, indicated by the (empty) third column
tetrahydronaphthalene branch (first child), having equal odds of being found in either set,
leads to an Ames-positive and an Ames-negative scaffold. A selection of the bicyclic rings
found is presented in Table 8.3 Such a two-way entry table may be useful for selection of
(bio)isosteric replacements with higher odds in the Ames-negative set. Similar tables can
be constructed for other properties.Ageneral finding from these data was that an increase in
aromaticity or extension of conjugation enhances the odds for mutagenic compounds. An
increase in the aliphatic character of rings decreases the mutagenic potential. To evaluate
the usefulness of the mutagenicity dataset (with a total of 6039 compounds), the authors
compiled a reference dataset consisting of 3882 commercially available drugs. Analysis
revealed that the chemical diversity within the mutagenicity dataset was significantly less
than the diversity of the marketed drugs. For the smaller drug set, 750 ring systems were
found, in contrast to the 427 ring systems found in the Ames-test dataset. The two sets had
199 ring systems in common.
Instead of studying a limited set of structural features such as ring systems,
[
37
]
others
have taken a more exhaustive approach. In such a scenario, all possible fragments are
examined to find those discriminative for a certain property, e.g. toxicity. Kazius
et al
. used
frequent fragment mining in order to derive toxicophores.
[
40
]
Similarly to Kho
et al
.,
[
37
]
structural elements were arranged according to mutagenic potential, thereby forming a
decision list. Most substructure mining methods use only part of the chemical information
in a molecule, viz. connectivity of the molecular graph (Figure 8.2), atom type labels and