Chemistry Reference
In-Depth Information
(a)
(c)
Time Domain Data, Fast Decay
Time Domain Data, Slow Decay
FT
0.10
0.20
0.30
0.40
s
0.10
0.20
0.30
0.40
s
Frequency Domain Data, Fast Decay
Frequency Domain Data, Slow Decay
(b)
(d)
1015
m/z
1015
m/z
1000
2000
3000
4000
m/z
1000
2000
3000
4000
m/z
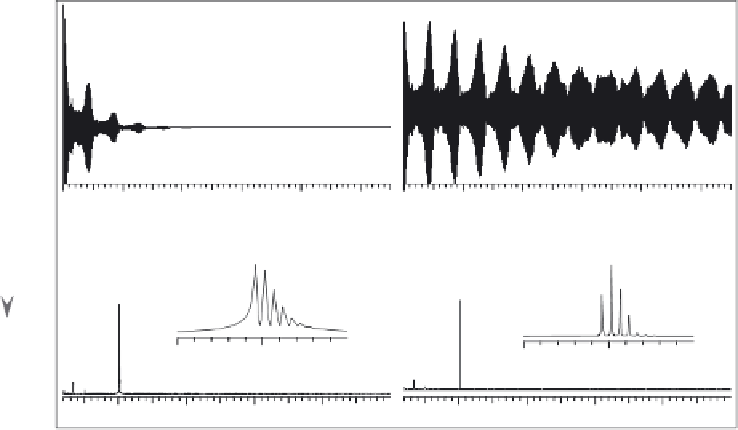
Figure 7.9
Fourier transformation of the time domain signal (a and b) to the frequency domain
signal and corresponding mass spectrum (c and d), showing the effects of signal decay time
on spectrum resolution and mass accuracy.
is much longer and excellent peak shapes and resolution can be achieve to generate low- or
sub-ppmmassmeasurement accuracy (compare Figure 7.9 entry awith b and entry cwith d).
A typical commercial FTMS system is pictured in Figure 7.10, with a schematic cut-
away showing the regions of differential pumping required to achieve the UHV conditions
in the mass analyzer cell. FTMS relies on high field superconducting magnets to achieve
its high-performance characteristics. The ability to achieve high resolution and isotopically
resolved spectra improves with increasing magnetic field strength so that higher field mag-
nets are preferred for this work. Commercial systems are now available with field strengths
up to 15.0 T, though 7.0, 9.4 and 12.0 T field strength systems are more common. Other
important characteristics of FTMS include its versatility in performing MS-MS experi-
ments and in obtaining highly accurate mass measurements. These last two features can
be of great benefit in determining the identity of the unknown binding species in noncova-
lent protein-ligand complexes.
[
49
]
Using well-established methods, a single protein adduct
(or several adducts) can be isolated in the FTMS mass analyzer cell and energized using
either a laser or collisions with an added neutral gas, leading to dissociation of the non-
covalent complex. The low mass ligands generated may then be observed and measured
with sufficient mass accuracy (typically low ppm) to determine elemental composition and
importantly the identity of library ligands. An example of the application of such high mass
accuracy measurements of binding ligands from carbonic anhydrase has been published
previously
[
50a
]
and an example of its application to a DCC study is shown later in this
chapter.
[
15
]
Thus the suite of tools available from FTMS make it an ideal instrument for
work with
in situ
medicinal chemistry as the fragments comprising the bound ligand can
be readily determined; this will be illustrated with examples later.