Chemistry Reference
In-Depth Information
(a)
(d)
9
+
(b)
(e)
34
+
35
+
20
+
10
+
8
+
800
1000
1200
1400
1600
m/z
3000 3200 3400 3600
m/z
29024.3
(c)
(f)
29024.3
28
600
29
000
29
400
m/z
28
600
29
000
29
400
m/z
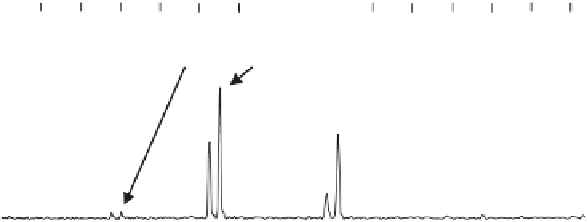
Enzyme Inhibitor
Complex
(g)
2300
2500
2700
2900
3100
3300
m/z
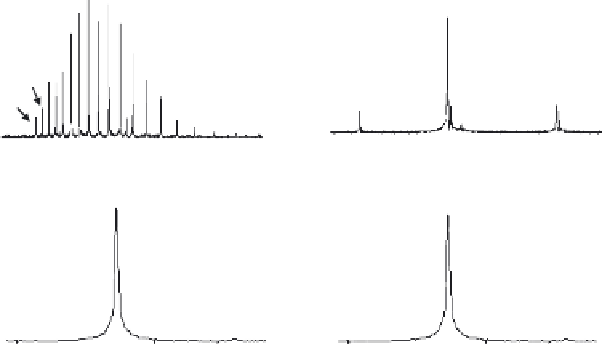
Figure 7.6
ESI of zinc metalloenzyme carbonic anhydrase, (a)-(c) under acidic denaturing
conditions, (d)-(f) native state conditions, and (g) native state conditions with a specific inhib-
itor. Structure of entry (d) is Protein Data Bank ID 1BN1. Boriack-Sjodin, P.A., Zeitlin, S.,
Chen, H.H., Crenshaw, L., Gross, S., Dantanarayana, A., Delgado, P., May, J.A., Dean, T.,
Christianson, D.W. Structural analysis of inhibitor binding to human carbonic anhydrase II.
Protein Sci
., 1998,
7
, 2483-2489.
molecular weight of the protein,
H
is the mass of the proton charge carrier and
z
is the
number of protons in a particular charge state, then the observed
m
/
z
for charge state
z
will
be (
MW
zH
)/
z
. If we use the experimentally observed
m
/
z
values for two adjacent charge
states
z
1
and
z
2
, observed at mass-to-charge ratios (
m/z)
1
and (
m/z)
2
, then we can write
two equations that can easily be solved for the molecular weight of the protein:
+
(m/z)
1
=
+
(MW
z
1
H)/z
1
(7.1)