Chemistry Reference
In-Depth Information
25
Hz
21
Hz
30
Hz
53
Hz
71
Hz
7.4
7.3
7.2
7.1
7.0
6.9
6.8
6.7
ppm
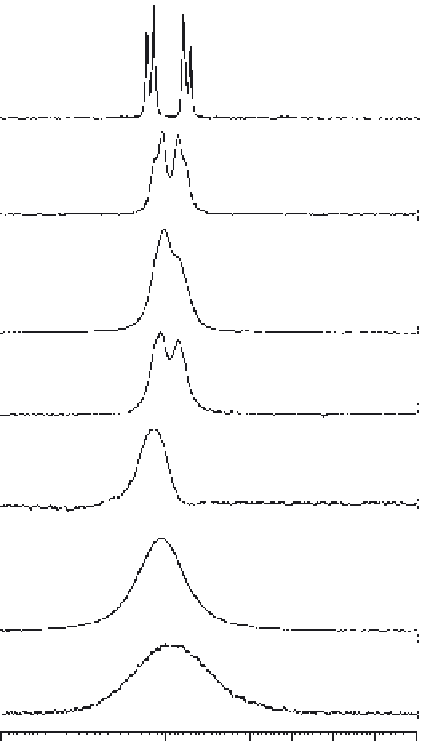
Figure 6.2
Effect of immobilization chemistry on the linewidth of compound s in solution. 1D
1
H
spectra of the aromatic protons of phosphotyrosine (pY) are shown with the fitted linewidth.
From top to bottom, pY in solution, in the presence of Actigel ALD, streptavidin Sepharose,
Zn-IDAA Sepharose, Zn-NTA Sepharose, Zn-NTA silica and controlled-pore glass beads (for
comparison).
At the pH at which we typically carry out immobilization (7.4), this reaction is fairly spe-
cific for the amino terminus. In principle, one could imagine that immobilization might
interfere with the functionality of certain proteins, such as kinases that contain a lysine
at an active site. Thus far we have not encountered this issue, but it is always possible to
block access to this lysine by immobilizing in the presence of high levels of an ATP mimic
such as AMPPNP. Kinases have been successfully immobilized for Biacore studies using
related chemistry.
[
18
]
We have investigated the use of IMAC resins to immobilize proteins
via a 6-His tag. Although this method is convenient, it is not possible to use Ni
2
+
as the ion
for chelating the tagged protein due to the potent paramagnetic relaxation. It is possible to