Chemistry Reference
In-Depth Information
same density as the target. The requirement for a reference protein comes from the fact
that TINS is highly sensitive to even very weak interactions between the compounds and
the immobilized target. Therefore, the choice of reference protein is important. Ideally, one
would like to have a reference proteinwhich is convenient to produce in large quantities, can
be readily immobilized, has the roughly 'typical' amounts of exposed surface charge and
hydrophobicity and has essentially no small-molecule binding capacity. The pH domain of
the cellular kinaseAKT is a nearly ideal candidate which we use for screening of all soluble
targets. Hajduk
et al
. showed that this protein was essentially refractory to small-molecule
binding using their well-known SAR by NMR assay.
[
16
]
Although we initially had concerns
that this small protein would be unrepresentative of larger, potentially multi-domain targets
or that proper cancellation of nonspecific binding would require accurate matching of total
surface area, this turns out not to be the case, as shown in Figure 6.1.
Immobilization is a constant source of questions with regard to TINS screening. In
principle, one is free to choose any immobilization approach which is compatible with
(a)
ADDITIVES
0.3% CHAPS
5% TFE
100 mM KSCN
K-based buffer
TRIS-based buffer
2 mM N-Octyl-Glucoside
1 mM N-Octyl-Glucoside
8
7
6
5
4
3
2
1
ppm
8
7
6
5
4
3
2
1
ppm
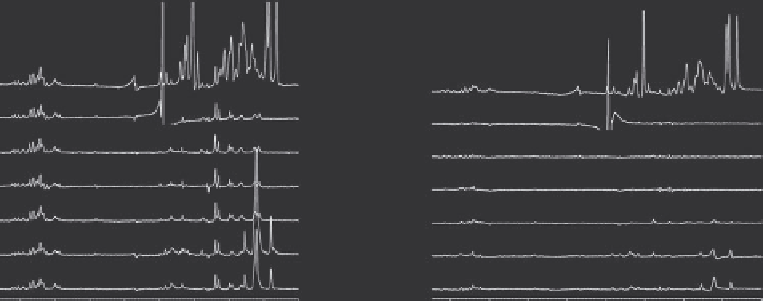
Figure 6.1
(A) Cancellation of nonspecific binding by the reference sample inTINS screening.
The left-hand panel shows difference
1
H
NMR spectra of a mixture of nonbinding compounds
acquired in the presence of Sepharose resin to which 6mgmL
−
1
of an SH2 domain (111 amino
acid residues) had been immobilized or just the resin itself. The indicated additive was included
with each of the compound mixtures. The right-hand panel shows the same difference spectra,
but the second spectrum was acquired in the presence of a resin to which 6mgmL
−
1
of FKBP
had been immobilized. The improvement in cancellation when an immobilized protein is used
as a reference is clear. (B) In this example, taken from a screen of a soluble target, both the target
and the reference protein (the pH domain of the kinase AKT) were immobilized on Actigel ALD
(Sterogene Bioseparations, Carlsbad, CA, USA) at a solution equivalent of 100 μM. A mix con-
sisting of three different compounds (upper three 1D
1
H
NMR spectra are of each compound
in the mix separately) was applied simultaneously to the sample of immobilized target and
reference protein in the dual-cell sample holder. Spatially selective Hadamard spectroscopy
1
was used to acquire simultaneously separate spectra of the compound mix in the presence of
the immobilized target and reference. These spectra are overlaid at the bottom of the figure. The
similarity of the two spectra indicates that none of the compounds specifically binds the target.
The weak interactions with any immobilized protein that are observed for most compounds in
the library are approximately the same for both the reference and target.