Environmental Engineering Reference
In-Depth Information
For flat plate electrodes a grid from hard lead (lead alloy with antimony or cal-
cium and further additives) carries the active material which is pasted into and
onto the grid. This design offers the advantages of a cost-efficient production and
high power densities. For tubular plate electrodes the active material is filled into
porous tubes around a central hard lead rod. This plate technology which is
mainly applied for the positive electrode and whose production costs are higher,
allows for considerably longer cycles lifetimes thanks to the good cohesion prop-
erties of the active mass. This electrode type is thus ideal for hybrid systems (PV
generator plus additional generators) with high energy throughput.
A further distinctive feature is the electrolyte status. The classic lead-acid bat-
tery is provided with a liquid electrolyte (so-called flooded batteries). The gases
produced within side reactions are directly emitted by the battery due to water
electrolysis (oxygen is emitted at the positive electrode, hydrogen at the negative
electrode; Fig. 6.23, left). This process consumes water, which must be regularly
refilled. In addition, battery rooms have to fulfil high requirements. Such rooms
need favourable natural or active ventilation to avoid critical hydrogen or oxygen
gas concentrations. Furthermore, electronic components and appliances must be
protected from the gases, since the latter have a corrosive effect when they are
moisturised by sulphuric acid.
O
2
H
2
O
2
geschlossene Batterie
mit flüssigem Elektrolyt
verschlossene
Gel or Vlies-Batterie
Vented battery with
liquid electrolyte
Valve-regulated
gel or AGM battery
Fig. 6.23
Gassing of batteries with liquid electrolyte (left) and valve-regulated batteries
with gel or AGM (absorptive glass mat) electrolyte (right)
As an alternative also so-called valve-regulated gel or AGM (absorptive glass
mat) batteries are available, which - instead of liquid sulphuric acid - contain gel
or AGM to adhere or to absorb the acid. These kinds of batteries enable the diffu-
sion of oxygen gas created at the positive electrode to the negative electrode, pass-
ing through micro-pores inside the gel or the glass mat separator. Since oxygen is
again reduced to water at the negative electrode no hydrogen is created here.
However, this only applies if all reactions are well balanced. If this is not the case,


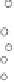
















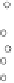






















Search WWH ::

Custom Search