Environmental Engineering Reference
In-Depth Information
-
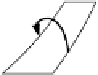
If the working medium is part of an "inexhaustible" reservoir (e.g. ambient air)
and its final state is different from the initial state, the process is referred to as
an "open cycle" (Fig. 5.4); yet, strictly speaking, such a process is also closed
since the last state change takes place outside of the actual process, namely
within the "inexhaustible" reservoir.
In the following such cycles are illustrated by means of temperature/entropy-
diagrams. These representations offer the advantage, that both isothermal (i.e.
constant temperatures) as well as isentropic (i.e. constant entropy) state changes
can be represented as straight lines (Fig. 5.4 (a)) /5-2/.
-
Within the Carnot cycle the entire exergy is extracted from the supplied heat so
that its full working capacity becomes useful. This cycle consists of isentropic
compression/decompression (i.e. performance of pressure change work) and
isothermal heat supply and dissipation. The Carnot cycle is an ideal compara-
tive process; however, mainly the isentropic compression/expansion cannot be
put into practice (Fig. 5.5 (a)).
-
The Ericson cycle represents the first technical approach to an ideal Carnot
cycle; isobaric compression and expansion substitute isentropic compres-
sion/decompression. Within this cycle addition and evacuation of heat is sup-
ported by internal heat transmission (Fig. 5.5 (b)).
-
The Stirling cycle is similar to the Ericson cycle. However, compres-
sion/decompression is isochore (i.e. density remains constant) (Fig. 5.5 (c)).
T
T
Added heat
Added heat
T
T
T
T
Θ
2
Θ
2
Θ
2
Θ
2
Θ
2
Θ
2
Useful work
Useful work
Θ
1
Θ
1
Θ
1
Θ
1
Θ
1
Θ
1
(a)
(a)
(b)
(b)
(c)
(c)
Removed heat
Removed heat
s
s
s
s
s
s
T
T
Θ
2
Θ
2
T
T
T
T
Saturation curve
Saturation curve
Saturation curve
Saturation curve
Θ
super
Θ
super
Θ
2
Θ
2
Θ
2
Θ
2
Θ
1
Θ
1
Θ
1
Θ
1
Θ
1
Θ
1
(e)
(e)
(f)
(f)
(d)
(d)
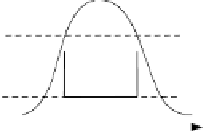
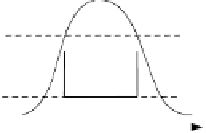
Fig. 5.5
Temperature/entropy diagram (
T,s
-diagram) of various cycles ((a) Carnot cycle,
(b) Ericson cycle, (c) Stirling cycle, (d) Joule cycle, (e) Clausius-Rankine cycle, (f) Clausi-
us-Rankine cycle with superheating) (
p
pressure,
V
volume,
T,θ
Temperature,
s
entropy)
s
s
s
s
s
s
-
The Joule cycle is composed of isentropic compression, isobaric heat addition
(combustion), isentropic expansion and isobaric heat dissipation (Fig. 5.5 (d)).













































































































Search WWH ::

Custom Search