Agriculture Reference
In-Depth Information
crops. The compounds are translocated from the point of uptake to the grow-
ing points where they inhibit normal cell growth. They are known to inhibit
lipid synthesis and auxin activity.
Toxicity
Several members of this group are formulated as emulsifiable concentrates
and oil in water emulsions. The inert ingredients can cause slight irritation to
eye, skin, and respiratory tract.
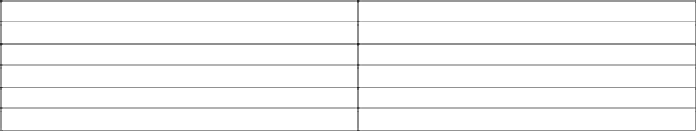
Table 5.20 Commercial products containing oxyphenoxy acid esters.
Active Ingredients
Trade Names
Diclofop-methyl
Hoelon 3EC,Illoxan
Fenoxaprop-ethyl
Acclaim, Horizon, Whip1EC
Fenoxaprop-p-ethyl
Option, Whip 360
Fluazifop-butyl
Fusilade 2000,
Fluazifop-p-butyl
Fusilade DX
p.
Substituted or Phenyl Urea Herbicides
These urea derivatives are a large group of important herbicides. The her-
bicidal efficacy of the first described member of this group, diuron, was shown
in 1951.
Most of the ureas are relatively nonselective and are usually applied to the
soil as preemergent herbicides; some have postemergent uses, while others are
active when applied to the foliage. Tebuthiuron is used on noncropland areas,
rangeland, rights-of-way, and industrial sites. Ureas are predominately ab-
sorbed by the roots and translocated to the rest of the plant.
Mode of Action and Toxicity
The mode of action of the substituted ureas is relatively well known. It
results in an inhibition of photosynthesis by blocking photosynthetic electron
transport and photophosphorylation.
Several members of this group are formulated as dry flowables, flowables,
water dispersible granules, and wettable powders. Improper application can
cause moderate irritation of the eye, skin, nose, and respiratory tract.
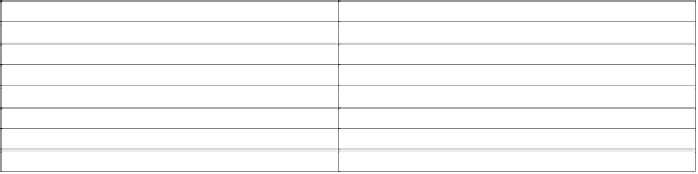
Table 5.21
Commercial products containing substituted ureas.
Active Ingredients
Trade Names
Diuron
Karmex, Seduron
Fenuron
Fenuron, Dozer
Fluometuron
Cotoran
Linuron
Lorox, Afalon
Siduron
Tupersan
Tebuthiuron
Spike
Thidiazuron
Dropp