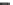Environmental Engineering Reference
In-Depth Information
Fig. 3.3 Molecular structure
of Celazole
(poly-2,2
0
-m-
(phenylene)-5,5
0
-
bibenzimidazole)
H
N
H
N
N
N
n
acids, are preferred due to their capability to act as both proton donor and acceptor,
they permit proton transfer by a dynamic process of breaking and forming of
hydrogen bonds [
25
]. These membranes present interesting features in terms of
proton conductivity, mechanical flexibility, and thermal stability; however, dura-
bility, start up time, and dynamic response are still critical issues, especially if
automotive applications are considered [
26
].
Apart from water uptake and proton conductivity, another important physico-
chemical property of a polymeric membrane for PEM fuel cells is its gas per-
meation, which can be considered a measure of membrane impermeability toward
reactant
species.
Permeability
is
defined
as
the
product
of
diffusivity
and
solubility:
P
¼
DS
ð
3
:
21
Þ
if D is expressed in cm
2
/s and S in mol/cm
3
/Pa, the permeability can be expressed
in mol cm/s/cm
2
/Pa, where cm represents the membrane thickness, the surface
area of the given material is expressed in cm
2
, mol/s is the gas flow rate passing
through the membrane at the pressure of 1 Pa. The most used unit for gas per-
meability is Barrer, being:
1 Barrer
¼
10
10
cm
2
=
s/cmHg
ð
3
:
22
Þ
An ideal membrane for PEM fuel cells should hinder the passage of other
species other than solvated protons, but due to material porosity and solubility of
hydrogen and oxygen in water, some reactant can actually permeate through the
membrane. For a dry Nafion membrane hydrogen permeability is comprised
between 20 and 70 Barrer in the temperature range 25-100C and 1 bar, whereas
oxygen permeability is about one order of magnitude higher, and both are higher
for a wet membrane [
27
]. Regarding the permeation rate, this is of course pro-
portional to permeability, pressure, and exposed surface of the membrane, and
inversely proportional to its thickness.
3.2.2 The MEA: Electrocatalysts
The electrodes in a PEM fuel cell have the fundamental function to provide a
support where the electrochemical reactions take place. As both the electrochemical
semi-reactions have to be catalyzed, to occur at temperatures under 90C, the