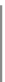Environmental Engineering Reference
In-Depth Information
Fig. 3.2 Molecular structure
of Nafion membranes. The
values of x, y, and z coeffi-
cients vary with the
manufacturer
CF
2
CF
CF
CF
2
2
X
Y
O
CF
2
FC
CF
3
Z
O
CF
2
CF
2
-
H
+
SO
3
In this type of membrane, as well as in similar products made by other man-
ufacturers, the Teflon-like backbone is responsible of very high chemical resis-
tance (due to the strong bond between carbon and fluorine), high hydrophobic
characteristics, and good mechanical properties. The hydrophobic feature is useful
to favor the expulsion of product water out of the cell, in order to prevent flooding
phenomena, while the mechanical strength permit the production of very thin films
(down to 50 lm).
On the other hand, the ionic bond between oxygen and hydrogen in sulfonic
groups favors a mechanism of clustering of side chains within the overall structure
of the co-polymer, due to the mutual attraction between protons and sulfonate
anions from different molecules. As sulfonic groups are highly hydrophilic, their
clustering inside a material substantially hydrophobic generates nano-domains of
strong affinity toward water molecules, which can significantly be absorbed by the
co-polymer up to 50% of its dry weight. An abundant collection of water mole-
cules around the hydrophilic regions creates large water reservoirs, where protons
result weakly bonded to sulfonate anions (dissociation of proton from sulfonic acid
is of course promoted by water) and able to move and transfer among neighboring
nano-domains, and then through the supporting long polymeric chain. This
mechanism of proton conduction is a mixture of a diffusion through water solvent
and proton skipping between the sulfonic acid groups. It is the most accepted
mechanism to explain the proton transport in Nafion membranes [
9
]. In order to
have a satisfying proton conductivity (at least 0.01 S/cm) the ratio of the number
of hydrophobic monomers to hydrophilic monomers has to be approximately
comprised in the range 3-7. The proton conductivity achieved in well-humidified
Nafion-like membranes can be as high as 0.2 S/cm at PEM fuel cell operative
conditions.
The necessity of adequate membrane hydration, with the associated risk of
drying out, limits the operative temperature of PEM fuel cells under 100C.