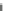Environmental Engineering Reference
In-Depth Information
Fig. 2.8
Phase diagram
T (K)
for H
2
10
4
H
2
gas
Critical point
Triple point
10
2
Solid H
2
Liquid H
2
1
10
-3
1
P (bar)
10
3
Table 2.2
Physical and thermodynamic data for automotive fuels
Property
Gasoline
Diesel
LPG
LNG
Hydrogen
Gas
350 bar
Gas
700 bar
Liquid
HHV (kJ/g)
47.5
44.8
50.3
55.5
141.9
141.9
141.9
LHV (kJ/g)
44.5
42.5
45.6
50.0
120.0
120.0
120.0
Gravimetric density
(kg/m
3
)
737
820-950
510
410-500
23.5
39.5
70.8
Energy density (MJ/L)
34.2
37.3
25.3
25.9
2.9
5.6
10.1
densities. The volumetric energy densities for hydrogen are calculated for liquid
and gas form at pressure values varying from 350 to 700 bars.
The analysis of Fig.
2.8
evidences that hydrogen has very low boiling point.
Liquid hydrogen exists only in a limited pressure-temperature region starting from
the triple point and ending at the critical point. A narrow temperature range
between 14 and 33 K is able to maintain the H
2
molecule in the liquid state in
dependence of the pressure value (varying from 0.07 to 13 bar). The saturated
liquid specific volume at atmospheric pressure is 0.014 m
3
/kg, while for H
2
gas in
standard conditions (273 K and 1 atm) it results 11.1 m
3
/kg.
Table
2.2
evidences that hydrogen has very low gravimetric and volumetric
energy densities. Hydrogen has the highest energy to weight ratio values (HHV
and LHV), in particular it has nearly three times the energy content of gasoline and
diesel fuels, but it contains less energy for a given volume when compared to the
other fuels. Liquid hydrogen does not reach a density close to that of typical
conventional liquid fuels, while hydrogen in gaseous form reaches a value of
volumetric energy density lower than hydrogen in liquid form also at a pressure of
700 bar.
In the last years, the scientific community has investigated another interesting
option regarding H
2
storage, based on the adsorption method [
107
-
109
]. The idea
is that a strong reduction of volumes should be obtained by interaction of hydrogen
with solid materials, in particular applying knowledge in gas-solid heterogeneous
process and exploiting recent advances of the material science applied to physical
and chemical adsorption.