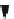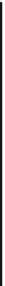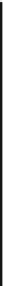Environmental Engineering Reference
In-Depth Information
(but also with carbon dioxide) to produce carbon monoxide and hydrogen,
according to the following equation that is the reverse of Eq.
2.8
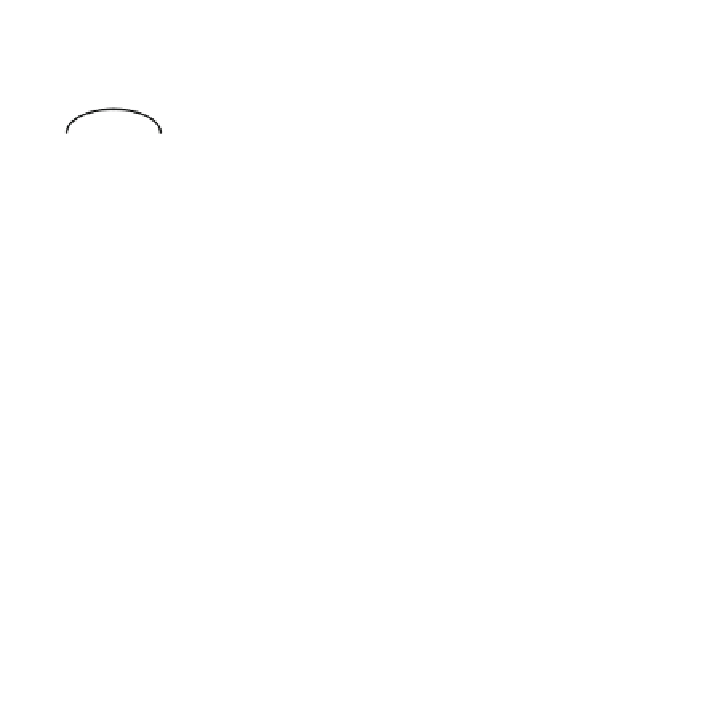
C
þ
H
2
OCO
þ
H
2
DH
¼þ
131 kJ/mol
ð
2
:
18
Þ
In addition, the reversible gas phase water gas shift reaction (
2.3
) reaches very
fast equilibrium at temperatures typical of a gasifier. The above chemical equa-
tions balance all the product (CO, CO
2
,H
2
O, H
2
) concentrations of the process.
Gasification process could be inserted in a Integrated Gasification Combined
Cycle plant (IGCC) [
54
,
55
] to improve the overall process efficiency. The syngas
produced in the gasifier is used as fuel in the gas turbine generator of the integrated
combined-cycle technology, which consists also of a heat recovery steam gener-
ator and a steam turbine/generator. A simplified scheme of a proposed gasification
overall plant for generation of both electricity and hydrogen is reported in Fig.
2.3
.
The scheme evidences different steps to produce electricity and hydrogen. The
heart of the overall process remains the gasifier. The coal fed to the reactor is
Sulfur By-Product
CO, CO
2
, H
2
Shift
Reactor
1000 - 1500
°C
H2
Solid By-product
Coal
Air
Air
Combustor
Compressor - Expander
Steam
Air
Gas Turbine
S
te
a
m Gen
e
rat
o
r
Solid By-Product
Electric Power
Electric Power
Steam Turbine
Fig. 2.3
Simplified scheme of an integrated gasification plant [
54
]