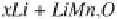Environmental Engineering Reference
In-Depth Information
which involves a cyclic transfer of lithium ions from the cathode (the lithium
source) to the graphite anode, with no metallic lithium present in the system. The
cell voltage is 3.8 V at room temperature.
This type of Li battery has already widely diffused in the electronic consumer
market, however for automotive applications the presence of a liquid electrolyte
is not considered the best solution in terms of safety, then for this type of
utilization the so-called lithium polymer batteries appear more convenient. They
are based on a polymeric electrolyte which permits the transfer of lithium ions
between the electrodes [
21
]. The anode can be composed either of a lithium
metal foil (in this case the device is known as lithium metal polymer battery) or
of lithium supported on carbon (lithium ion polymer battery), while the cathode
is constituted by an oxide of lithium and other metals, of the same type used in
lithium-ion batteries, in which the lithium reversible intercalation can occur. For
lithium metal polymer batteries the overall cycling process involves the lithium
stripping-deposition at the anode, and the deintercalation-intercalation at the
anode, according to the following electrochemical reaction, written for a Mn-
based cathode:
ð
5
:
22
Þ
The absence of liquid phases facilitates the construction of leak-proof and light-
weight containers, which represents an additional advantage for automotive
applications. In particular it is possible to realize sandwich of foils at low cost,
with the great advantage on the packaging flexibility and insensitivity to shock and
vibration damage, as required by users.
Recent developments in the field of lithium batteries have been focused on the
possibility to reach very high energy and power densities by using new types of
anode and cathode. Metals and semiconductors, such as Al, Si, Sn, Bi, have been
considered for their capacity to form alloys with lithium, which are characterized
by a theoretical charge capacity very higher than traditional carbon materials
(in particular, a Si-Li alloy presents a theoretical specific capacity of 4200 mAh/g,
to be compared with 371 mAh/g of graphite [
22
,
23
]). However, the large volume
change associated with the formation of the alloys (by lithium intercalation/de-
intercalation) rapidly leads to electrode pulverization, strongly limiting the cycling
capability of the battery [
23
]. To overcome this type of limitation different solu-
tions are under study, from reduction of metal particle size down to nanoscale
[
24
,
25
] to utilization of composite materials (in which an inactive component
added to the active metal acts as a buffer for volume variations [
26
,
27
]) or metal
hydrides as anode [
28
]. The researches about the cathode of lithium ion batteries
are intensively oriented on high voltage spinels and high capacity layered lithium
metal oxides [
29
-
32
].