Agriculture Reference
In-Depth Information
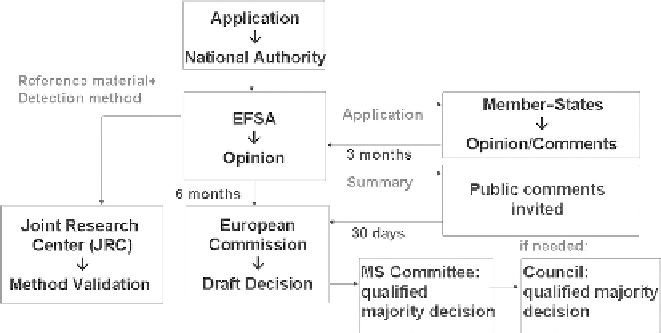
Figure 1. Authorization procedure for GM food and feed in the European
Union.
risk assessment is conducted, and a single authorization is granted, for a
GMO and its possible uses. GMOs likely to be used as food and feed can
only be authorized for both uses, or not at all. However, cultivation of
GMO or commercialization of GMO for nonfood or nonfeed purposes
still needs an authorization in accordance with Directive 2001/18/EC.
Authorizations are limited to a ten-year period but are renewable.
Applications for renewals of authorizations for products that have been
lawfully placed on the Community market before Regulation (EC) No
1829/2003 entered into force are required within nine years from the date
of which the products were first placed on the market.
The assessment of environmental risks as well as the safety assess-
ment of GM food and feed is no longer the responsibility of member-
states but of EFSA, whose opinions are made available to the public
with the opportunity to make comments. CAs of member-states are also
invited to provide comments. Based on an EFSA opinion, the European
Commission drafts a proposal for granting or refusing authorization. A
Standing Committee of Representatives of member-states (MS Commit-
tee) then decides whether to accept the Commission's proposal through a
weighted voting system. If the committee's proposal is neither accepted
nor rejected by a qualified majority of member-states, it is referred to