Biology Reference
In-Depth Information
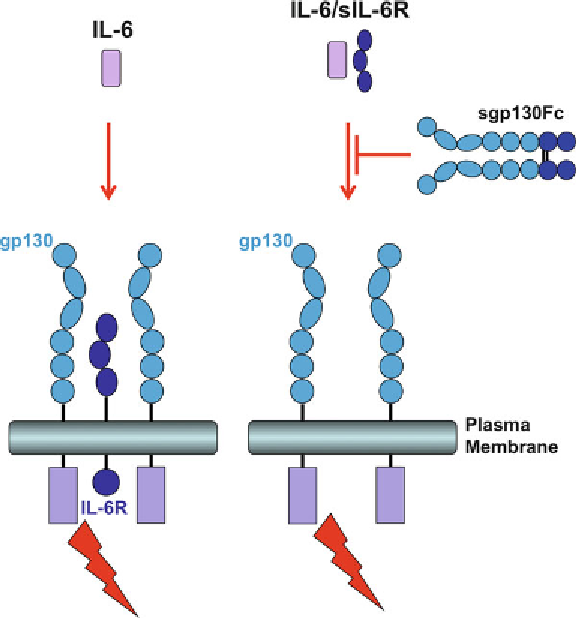
Fig. 3
The sgp130Fc fusion protein specifically blocks IL-6 trans-signaling. IL-6
stimulation of cells expressing membrane bound IL-6R is not affected by
sgp130Fc (
left
), whereas IL-6 trans-signaling is efficiently blocked by sgp130Fc
(
right
)
3
Signal Transduction by IL-6
As reviewed in (
5, 6
), signaling of IL-6 occurs upon dimerization of
gp130 and activation of JAK kinases constitutively associated with the
cytoplasmic portion of gp130. This leads to auto-phosphorylation
of JAK kinases and subsequently to phosphorylation of five tyrosine
residues within the cytoplasmic domain of gp130. The juxtamem-
brane tyrosine (Y759 in human gp130) leads to the activation of
SHP2 and in turn to the activation of the ras/ERK and the
PI3K/AKT pathways (
5, 6
). The other four tyrosine residues of
gp130—when phosphorylated—lead to the recruitment of STAT
factors, namely STAT1 and STAT3; these become phosphory-
lated, dimerize and translocate into the nucleus, where, as
homodimers or heterodimers, they act as transcription factors
and activate the transcription of STAT target genes (
5, 6
).
Search WWH ::

Custom Search