Biology Reference
In-Depth Information
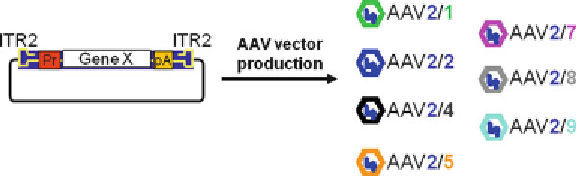
Fig. 2
Versatility of AAV vector production. The picture shows a schematic repre-
sentation of a
cis
plasmid required for AAV vector production. The
cis
plasmid
contains the expression cassette encoding for the gene of interest flanked by the
ITRs from AAV2. The availability of a different capsid from each serotype and the
versatility of the AAV production system allow to easily exchange capsids among
various AAV serotypes thus creating hybrid vectors containing a genome with the
same ITRs (e.g., from AAV2) and the capsid from a different serotype (
54
) . Vectors
obtained through this trans-capsidation system are named AAV2/
n
, where the first
number refers to the ITRs and the second to the capsid. Each AAV serotype has its
own transduction characteristics (i.e., target cells, kinetics of transgene expres-
sion) and the user can choose the most appropriate for the cell/organ of interest
(
55
) .
Pr
promoter,
pA
polyadenylation signal sequence,
gene X
gene of interest
3.1.2 Generation
of the pAAV2.1-CMV-EPO
Plasmid
1. PCR-amplify the full-length coding sequence (CDS) of the
murine EPO gene using as template murine cDNA, a forward
oligonucleotide containing the NotI restriction site and a
reverse oligonucleotide containing the BamHI restriction site.
2. Recover the amplicon of the expected size by gel extraction.
3. Sequence the amplicon to be sure that it corresponds to the
EPO CDS and it does not contain mutations.
4. Digest the pAAV2.1-CMV-EGFP3 plasmid (
48
) using NotI
and BamHI restriction enzymes to remove the EGFP CDS
and purify the fragment of interest by gel extraction.
5. Digest the amplified EPO CDS using NotI and BamHI restric-
tion enzymes and purify the fragment.
6. Ligate the EPO CDS and the digested pAAV2.1-CMV-EGFP3
backbone fragments following standard procedures.
7. Select the clone of interest using standard bacterial transforma-
tion and selection methods.
8. Once the clone pAAV2.1-CMV-EPO has been obtained, make a
large plasmid preparation using endotoxin-free solutions and
materials (e.g., Endofree Plasmid Giga kit, Qiagen, S.P.A., Italy).
1. Mutagenize the EPO CDS following the protocol contained
in the Quick Change II XL Site-Directed Mutagenesis (Agilent
Technologies, USA) datasheet. Use the pAAV2.1-CMV-EPO
3.1.3 Generation of
the pAAV2.1-CMV-S100E
Plasmid
Search WWH ::

Custom Search