Biology Reference
In-Depth Information
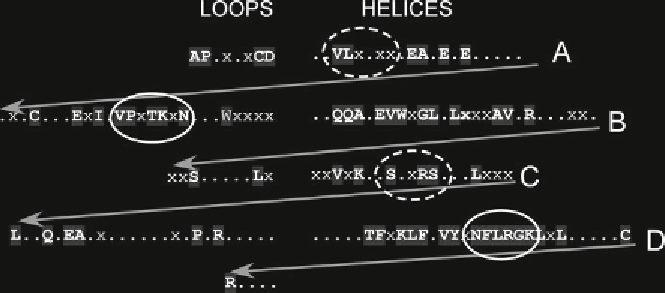
Fig. 3
Helix B of the EPO molecule is highly conserved over 360 million years of natural selection. Homology
between human and amphibian (
Xenopus laevis
) EPOs: helices are aligned to the
right
and
arrows
indicate
continuation of the protein sequence into the loops adjacent to the helices (
left
).
Shaded uppercase bold letters
correspond to identical amino acids, whereas
x
represents strong conservative substitutions. Binding sites to
the hematopoietic receptor (
solid ellipse
high af fi nity;
dashed ellipse
low affinity) as well as helix B are highly
conserved (N-terminal is at the
upper left
and C-terminal at the
lower left
)
comparing the sequence homology of evolutionarily distant verte-
brates. For example, an EPO molecule has been recently identified
from
Xenopus laevis
, the African clawed frog, that shared a com-
mon ancestor with humans approximately 360 million years ago
(
22
). Examining for sequence similarities, a number of regions of
the EPO molecule are highly conserved (Fig.
3
). Most of these
correspond to the regions of EPO that bind to the dimeric
hematopoietic receptor. However, helix B, which does not bind to
the hematopoietic receptor, is also highly conserved, consistent
with an important biological function requiring conservation of
structure to maintain function. We hypothesized that this region
bound to the tissue protective receptor.
To test the hypothesis that the tissue protective activity of EPO
resided within helix B, a 25 amino acid peptide constituting the
sequence of helix B was synthesized (
21
). As expected, this peptide
was not erythropoietic. However, it was fully tissue protective in a
wide variety of in vitro and in vivo animal models (
21
). Additional
consideration of the molecular structure of EPO led to a
simplification of the structure needed for tissue protection. Namely,
as previously noted EPO is a globular molecule—the helices inter-
act to provide structure for the molecule. Therefore, a specific por-
tion of helix B always faces out into the aqueous medium (Fig.
2
).
Utilizing crystallographic data, the specific amino acids that are on
the aqueous face of helix B were identified and a small 11 AA pep-
tide was constructed, termed Helix B Surface Peptide (HBSP).
This peptide was fully tissue protective in a wide variety of models
of tissue injury (
21
), confirming the importance of helix B in the
endogenous response to injury.
Search WWH ::

Custom Search