Environmental Engineering Reference
In-Depth Information
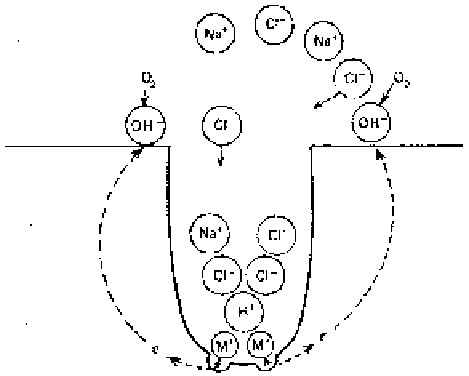
Figure 3.10
Growth of a corrosion pit.
more in the direction of gravity. In 304 stainless steel, chromium and nickel
provide additional hydrolysis reactions similar to Eq. 3.4 and more of acidic
condition is produced. Thus the 304 stainless steel is more susceptible to pitting
damage than the carbon steels. However, stainless steels containing higher
amounts of nickel and chromium or with molybdenum addition (type 316) show
high resistance to pitting, apparently because of a more stable passive surface
produced by these alloying additions.
3.4.4
Remedial Measures
1.
Solution aggressiveness can be reduced by eliminating chlorides or by de-
creasing its concentration.
2.
Stagnation of the solution should be avoided.
3.
Addition of passivating inhibitors helps in reducing or eliminating pitting
attack. However, they should be used in sufficient amount to ensure complete
passivation. Otherwise, the attack will aggravate (''dangerous inhibitor,''
Section 4.2.3.1).
4.
Cathodic protection, through sacrificial anode or by impressed current, has
been found to prevent pitting in marine applications (i.e., 304 stainless steel
propellers). However, this may not work out in more aggressive solutions.
5.
Using a metal or alloy of higher resistance to pitting is often a practical
solution, but with increase in investment cost which may prove to be econom-
ical in the long run. A stainless steel with 2-3% molybdenum (type 316) is