Environmental Engineering Reference
In-Depth Information
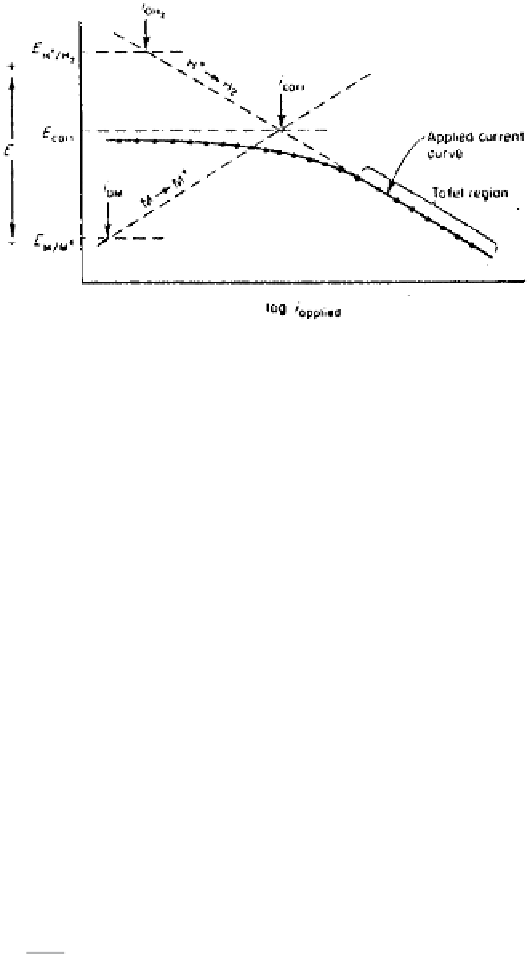
Figure 2.27
Tafel extrapolation method of corrosion rate measurement through ca-
thodic polarization.
also affected. These disadvantages are largely overcome in the linear polarization
method.
Linear Polarization Method
It is observed that within 10 mV more noble or more active than the corrosion
potential, the applied current density is a linear function of the electrode potential.
This is shown in Fig. 2.28. The slope of this linear polarization curve is given
by [8]:
∆
E
β
a
β
c
2.3(
i
corr
)(
(2.49)
i
app
∆
β
a
β
c
)
where
β
c
are Tafel slopes for anodic and cathodic reactions, respectively.
The slope has its unit in ohms and is referred to as
polarization resistance
,
R
p
.
Hence the method is also known as the polarization resistance method. Although
the linearity of the curve deviates at higher overvoltages, the slope of the curve
at the origin is independent of the degree of linearity. The slope of the linear
curve is thus seen to be inversely proportional to the corrosion, current,
i
corr
.
Assuming
β
a
and
β
a
β
c
0.12 V, Eq. 2.48 reduces to:
∆
E
0.026
(
i
corr
)
i
app
(2.50)
∆
One can calculate the corrosion rate from this equation without knowledge of
the kinetic parameters. This principle has been utilized in devising commercial
instruments for corrosion rate measurement. Such instruments are based on galva-