Environmental Engineering Reference
In-Depth Information
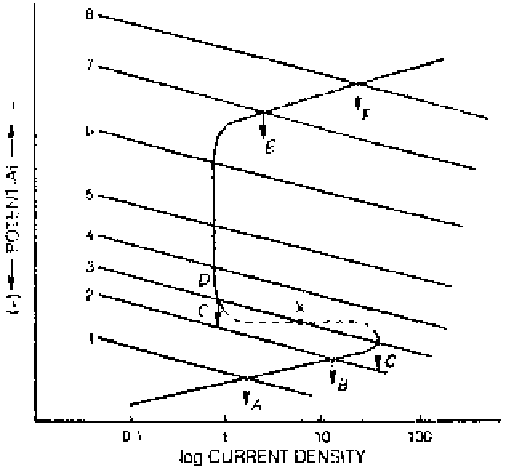
Figure 2.24
Effect of oxidizer concentration on the corrosion behavior of an active-
passive metal.
or Pd with corroding titanium or chromium brings down the corrosion rate
drastically, whereas such a coupling with corroding iron enhances corrosion.
A similar effect is obtained by noble metal alloying of titanium or chromium
where after an initial corrosion the surface is left with islands of noble metals
on the base metal matrix causing, in effect, a galvanic coupling.
The situation here is effecting an increase in the cathodic exchange current
density and its subsequent effect on the polarization behavior, as depicted in
Fig. 2.25.
The exchange current density for hydrogen evolution on platinum or palladium
is much higher than that on titanium or chromium, and the polarization is also
sluggish. That is why the cathodic polarization curve initially intersecting the
anodic curve in the active region intersects it in the passive region after galvanic
coupling and the corrosion rate decreases. In the case of iron, however, the pas-
sive region is beyond the redox potential of hydrogen evolution reaction. There-
fore, the increased exchange current density and a flatter cathodic polarization
curve on galvanic coupling enhances the corrosion rate because of an intersection
at a higher current density value in the active region (Fig. 2.26).