Environmental Engineering Reference
In-Depth Information
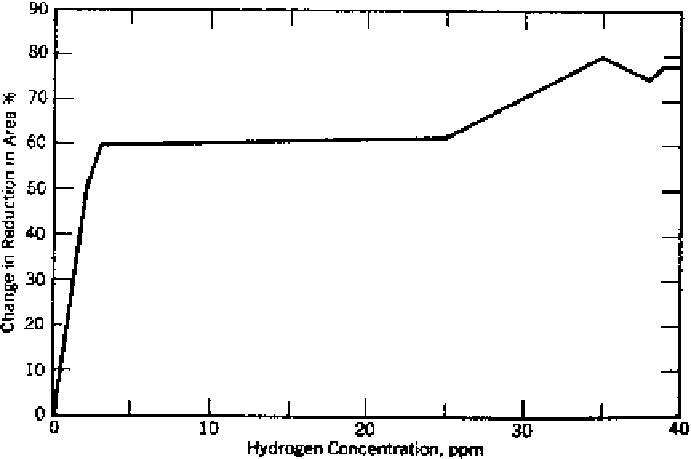
Figure 8.1
Loss of ductility in steel as a function of hydrogen content [23].
This type of damage is most often observed in lower strength alloys and has
been encountered in steels, stainless steels, nickel-base alloys, aluminum alloys,
and titanium alloys exposed to hydrogen. Figure 8.2 shows the ductility loss for
several austenitic stainless steels in high-pressure hydrogen. A wide variation in
hydrogen damage in these alloys is apparent. Type 304L is most susceptible and
the stable austenitic alloys, such as 15Cr-25Ni, are minimally affected.
The loss of ductility is temporary and can be reversed by the driving out of
hydrogen from the metal. This is accomplished by heating the metal. The rate
of recovery depends on time and temperature; lower is the time required at higher
temperatures. However, heating above 315
C is not usually recommended due
to the risk of high-temperature hydrogen attack.
°
Hydrogen Stress Cracking
Hydrogen stress cracking (HSC) refers to the brittle fracture of a normally ductile
alloy under sustained load in the presence of hydrogen. This type of damage has
been encountered in carbon and low-alloy steels, stainless steels, nickel alloys,
and aluminum alloys. HSC has been studied most extensively in steels and has
been described by various other names, i.e., hydrogen-induced cracking (HIC),
hydrogen-assisted cracking (HAC), delayed failure, and static fatigue. The cata-