Environmental Engineering Reference
In-Depth Information
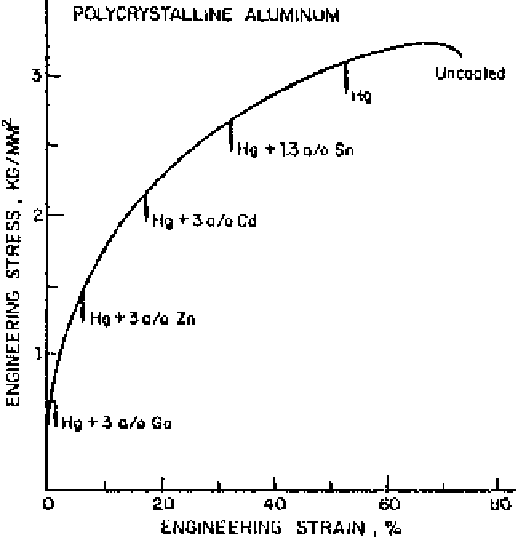
mercury solutions is shown in Fig. 7.5. From the observations made in various
systems it can be concluded that (1) the maximum embrittlement occurs when
the solid metal and the embrittling metal have the similar electronegativity, and
(2) the degree of embrittlement induced in the solid metal decreases as the differ-
ence in electronegativity between the metals of the couple increases.
The quantitative definition of electronegativity is the power of an atom in a
molecule to attract electrons to itself. The difference in electronegativity is a
measure of the tendency for two elements to form ionically bonded compounds.
For the metals with the similar electronegativity such affinity is the least, which
means that there will be little mutual solubility. Apart from this support for the
empirical rule stated earlier, the fundamental significance of the correlation be-
tween the severity of embrittlement and electronegativity has not been under-
stood.
Figure 7.5
Embrittlement of polycrystalline pure aluminum by various mercury solu-
tions [4].