Environmental Engineering Reference
In-Depth Information
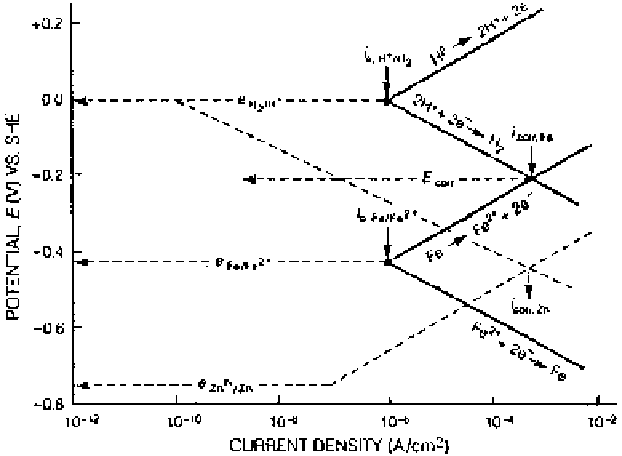
the rate of corrosion depends on exchange current density and Tafel slope values.
An example will clarify this.
In the emf series, the redox potential for zinc is more negative (
0.76 V) than
that for iron (
0.44 V). In an acid solution, however, the corrosion rate of iron
is greater than that of zinc. The higher values of exchange current densities for
dissolution of iron lead to this behavior. Graphically, the situation is represented
in Fig. 2.16.
The effect of the addition of an oxidizing agent on the corrosion rate can also
be appreciated clearly through graphical representation of kinetic behavior. For
example, the corrosion rate of zinc in hydrochloric acid increases in the presence
of ferric chloride. Two reduction reactions, those of ferric ions and hydrogen
ions, are active in this case and the total reduction rate equals the corrosion rate
according to:
i
corr
i
(Fe
3
→Fe
2
)
i
(H
→H
2
)
(2.43)
In the graphical representation shown in Fig. 2.17, the resultant cathodic polar-
ization is shown by dotted lines. It is to be noted that
i
corr
corr
and
E
corr
has also
shifted slightly. Rate of hydrogen evolution shows a decrease from a value of
i
i
corr
to
i
H
→H
2
although the overall increase in corrosion rate has resulted from the
additional cathodic reduction reaction.
Figure 2.16
Comparison of kinetic behavior of iron and zinc in acid solution.