Environmental Engineering Reference
In-Depth Information
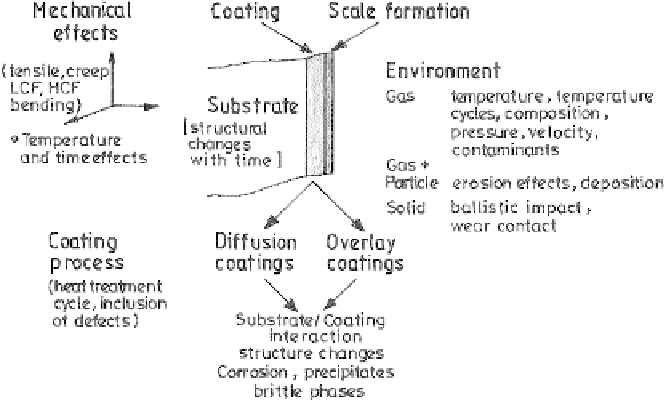
Figure 6.45
Interactions between coating, substrate, and environment [68].
ZrO
2
, HfO
2
, ThO
2
, and Cr
2
O
3
(as single oxide), their complex oxides, spinels,
and so forth. Of these, CaO and La
2
O
3
hydrate rapidly in air; BeO is markedly
toxic; and pure ZrO
2
undergoes polymorphic transformations. However, this tran-
sition can be eliminated by stabilizing ZrO
2
in a cubic form with the addition of
other oxides, such as CaO, MgO, and Y
2
O
3
. It is pertinent to point out that most
refractory oxides undergo chemical reactions among themselves at temperatures
well below their individual melting points, with the formation of low-melting
eutectic liquids. Accordingly, the useful temperature range of their applications
becomes limited. Cr
2
O
3
is stable only at temperatures below 1273 K in atmo-
spheric oxygen pressure.
Diffusion in Oxides
For a protective oxide film, its effectiveness in combating further degradation of
the underlying metal/alloy is usually determined by the rate of solid-state diffu-
sion through the film. The most effective diffusion barriers are provided by oxides
having the slowest rates of diffusion of the reactants. Accordingly, it is essential
to have knowledge about the diffusion rates of both cations and oxygen. A com-
parative plot of self-diffusion coefficients of cations and oxygen in some simple
oxides of interest [2] is illustrated in Fig. 6.46. This figure clearly demonstrates
that oxides like CaO, MgO, and Al
2
O
3
, which exhibit small deviations from stoi-
chiometry, have the smallest diffusion coefficients. On the other hand, oxides like