Environmental Engineering Reference
In-Depth Information
In an oxidizing environment, a metallic material may be protected from degra-
dation in two ways: by alloying with suitable elements or by coating. In either
case, the objective is the same, i.e., to form or obtain a layer on the metallic
surface that acts as a barrier separating the two reactants (the underlying metallic
substrate and reacting gas), thereby minimizing the reaction between them. The
high-temperature corrosion resistance of numerous alloys in practice is provided
by scales consisting of chromia, alumina, and silica or more complex oxides of
these, e.g., various spinels. Such scales are achieved by preferential or selective
oxidation of chromium, aluminum, or silicon present as constituents of the alloys
or coatings. The oxide usually preferred at less than 1273 K is Cr
2
O
3
(because
at such temperatures volatile CrO
3
formation becomes appreciable at or near at-
mospheric pressures), whereas Al
2
O
3
and SiO
2
are chemically more stable at
higher temperatures.
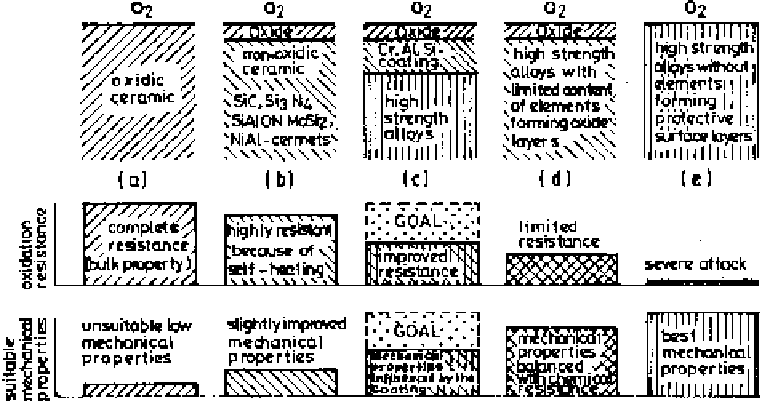
Formation of such oxide layers can be considered in various ways [66], as
depicted in Fig. 6.44. In the consideration of coatings, it is important to recognize
the coating and the underlying substrate as an integral system under the operating
conditions of the component. Prefabricated oxides can be developed with maxi-
mum chemical inertness as shown in Fig 6.44a, but they usually suffer from
unfavorable mechanical properties, such as brittleness. Moreover, in most such
cases these are physically incompatible with the metallic substrate. If chemical
Figure 6.44
Schematic illustration of the possible situations for protective oxide layer
formation rendering oxidation resistance and mechanical properties of metallic materials
at high temperatures [66].