Environmental Engineering Reference
In-Depth Information
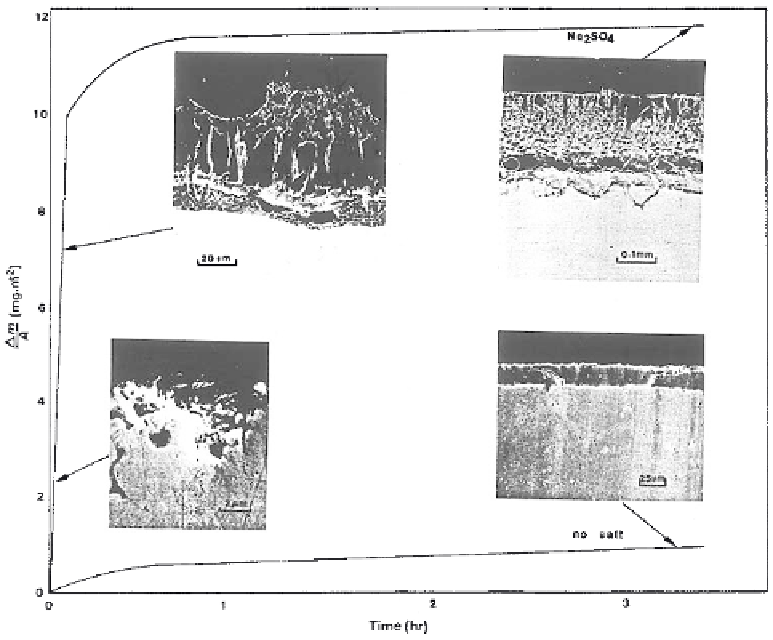
Figure 6.37
Mass change versus time for pure Ni with and without Na
2
SO
4
deposit
and associated scale microstructures [16].
Equation (6.35) further suggests that with increasing consumption of SO
2
and
O
2
, the oxide ion (O
2
) activity in the melt will increase to maintain equilibrium.
As a result, the salt will turn more basic in nature. The increase in basicity will
reach a maximum at the areas of sulfide formation, i.e., where SO
2
is consumed
most rapidly. In these (Fig. 6.38b), the NiO scale will react to form soluble nick-
elate ions (NiO
2
) according to Eq. (6.38). These, in turn, will diffuse to the salt-
gas interface where the oxide ion concentration is low and will allow the liquid
salt to penetrate it and be spread along the scale-metal interface (Fig. 6.38c),
lifting and cracking the scale. It is possible that such cracking is initiated by the
formation of a liquid Ni-S phase at the scale-metal interface with a greater molar
volume than that of nickel. The cracking of the scale allows oxygen penetration,
resulting in oxidation of the sulfides and freeing of sulfur to further penetrate