Environmental Engineering Reference
In-Depth Information
6.7.2 Kinetics
Several authors have suggested that hot corrosion of all susceptible alloys and
coatings is characterized by a two-stage process: an incubation period with a
slow rate of reaction, possibly with the alloy being protected by a stable Cr
2
O
3
or Al
2
O
3
layer; and a propagation stage with rapid and often catastrophic material
degradation as illustrated in Fig. 6.32. During the incubation period, elements in
the alloy are oxidized, and electrons are considered to be transferred from metallic
atoms to reducible substances in the salt deposit. When the reduced substances
are the same as those that would have reacted with the alloy in the absence of
the deposit, the reaction product barrier forms beneath the salt on the alloy sur-
face. As the hot corrosion process is continued, certain features become apparent
which indicate that the salt is affecting the corrosion process, and eventually the
selective oxidation process is ineffective. The time, which may vary from a few
hours to thousands of hours for which the most effective reaction product barrier
is stable beneath the salt layer, is influenced by a number of factors as illustrated
in Fig. 6.33. These factors include alloy composition and its microstructure, fabri-
cation condition, pretreatment of alloys, environment composition, gas velocity,
erosion, salt deposit condition and its composition, performance/test temperature,
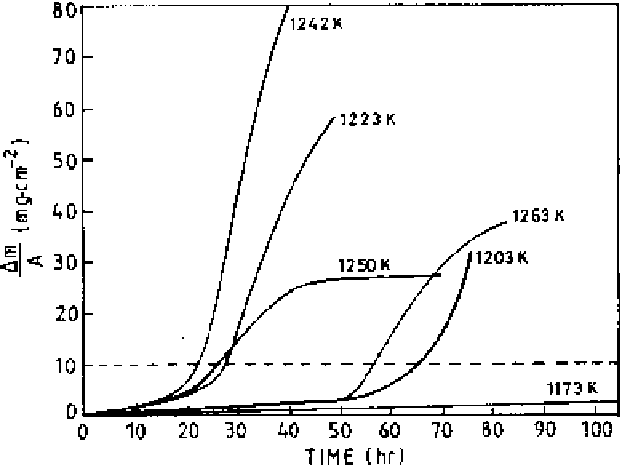
Figure 6.32
Mass change versus time for IN-738 alloy coated with 1 mg/cm
2
Na
2
SO
4
in 1 atm O
2
at different temperatures [16].