Environmental Engineering Reference
In-Depth Information
In Fig. 6.27, the activity of Na
2
O is considered to be unity at the Na
2
O/Na
2
SO
4
boundary. At sufficiently low oxygen and sulfur activities (not shown in the fig-
ure), Na
(l)
is the only stable phase in the Na-O-S system; otherwise Na
2
O is the
only stable phase at sufficiently low sulfur activities and Na
2
S at sufficiently low
oxygen activities.
For interpretation of the reactions of a metal in a mixed environment consisting
of two oxidants such as sulfur and oxygen, one needs to consider the phase stabil-
ity diagram of the corresponding metal-oxygen-sulfur system also at the temper-
ature under consideration. Such diagrams define the stability regions of various
phases that may exist at different activities of the two oxidants. These are the
high-temperature analogs of well-known Pourbaix diagrams used in aqueous cor-
rosion processes.
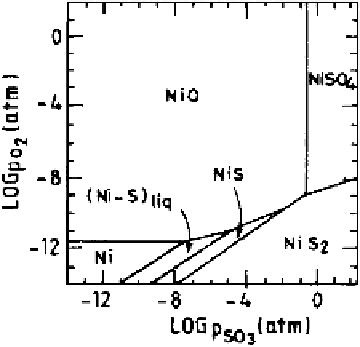
As an illustration, the phase stability diagram for the Ni-O-S system at 1173
K is presented in Fig. 6.28 in terms of activities of O
2
and SO
3
, where activity
of nickel has been considered equal to unity [50]. This diagram illustrates that
at sufficiently low activities of both O
2
and SO
3
,Ni
(s)
metal is the only stable
phase; at low SO
3
activities and high O
2
activities NiO is the only stable phase.
While at high activities of both O
2
and SO
3
, NiSO
4
is the stable phase. The
possible sulfide phases at this temperature are Ni-S liquid solution, NiS, and NiS
2
.
It is to be recognized that such diagrams do not provide any information about
the mutual solubility of the phases, e.g., solubility of oxygen and sulfur in nickel
metal, sulfur solubility in NiO, etc.
To interpret the Na
2
SO
4
-induced hot corrosion process of nickel, it is neces-
sary to know how the presence of Na
2
SO
4
affects the Ni-O-S phase stability
Figure 6.28
Phase stability diagram for the Ni-S-O system at 1173 K [50].