Environmental Engineering Reference
In-Depth Information
A wide number of reactive elements or their oxide dispersion additions pro-
duce all or most of the above-mentioned effects but to differing degrees. The
main critical factor is that a potential reactive element must have a higher affinity
for oxygen than the element that is to form the protective scale. The effect of
adding a reactive element to an alloy is more or less the same as that of adding
a stable oxide dispersion since the reactive element oxidizes internally ahead of
the scale-alloy interface.
6.6.3 Proposed Mechanisms
Scale Growth Mechanisms
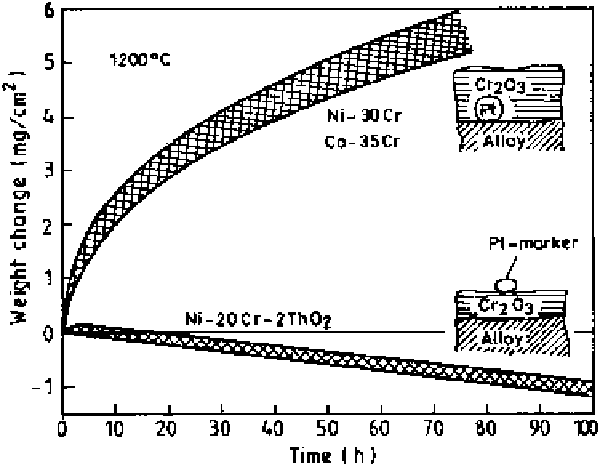
The prominent effects of RE additions on scale growth processes for Cr
2
O
3
-form-
ing alloys are illustrated in Fig. 6.22, which compares the oxidation rates for
TDNiCr (Ni-20%, Cr-2vol%, ThO
2
) to Ni-30% Cr and Co-35% Cr alloys at
1200
C [50]. The platinum markers were found to be situated at the alloy-scale
interface for the binary alloys without any active element/oxide additions, and
at the scale-gas interface for the thoria dispersed alloy. This clearly suggests that
outward chromium diffusion is the important process for the binary alloys,
whereas inward oxygen diffusion is important for the thoria dispersed alloy. The
°
Figure 6.22
Comparison of the oxidation of Ni-30%Cr, Co-35%Cr, and TDNiCr (Ni-
20%Cr-2vol%ThO
2
) at 1200
°
C [50].