Environmental Engineering Reference
In-Depth Information
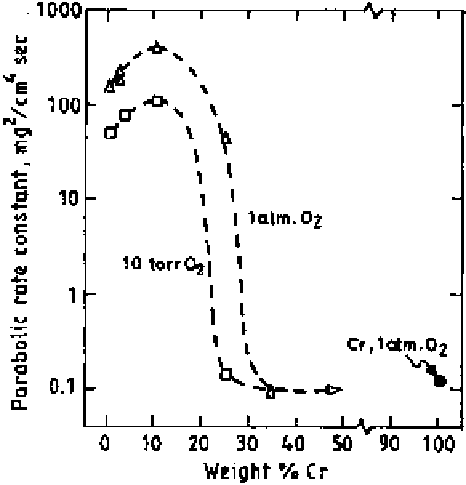
Figure 6.16
The parabolic rate constant for oxidation of pure Cr and Co-Cr alloys at
1373 K as a function of Cr content [32].
3.
Co-Cr alloys—CoO, Co
3
O
4
(at T
1243 K in 1 atm O
2
), CoCr
2
O
4
, and
Cr
2
O
3
It is possible for FeO, NiO, and CoO to dissolve a low percentage of Cr (1.5-
2% in FeO at 1273 K, 1.45% in NiO at 1273 K, and 2.6% in CoO at 1473 K
[30]) to form solid solutions and similarly for Cr
2
O
3
to dissolve some Fe, Ni, or
Co (doped oxide). The native cation vacancy levels in pure NiO, CoO, and FeO
are approximately 0.1, 1, and 10%, respectively in 1 atm O
2
at 1273-1473 K
[30]. It is expected that doping at the concentration levels possible would be
more effective in the more nearly stoichiometric oxides; its importance in increas-
ing the cation vacancy level should decrease in the order NiO
FeO.
The likelihood of a cation vacancy concentration gradient also lies in this order.
When Fe-Cr, Ni-Cr, and Co-Cr alloys are oxidized, the different oxides as
mentioned above are produced on the respective alloy in various proportions and
locations and at different exposure timings, depending on conditions such as alloy
composition, impurities, atmosphere, temperature, alloy surface geometry, sur-
face finish, etc. Among the three binary alloy systems, Fe-Cr alloys are ferritic
(BCC), whereas Ni-Cr and Co-Cr alloys are austenitic (FCC). Accordingly, it is
CoO