Environmental Engineering Reference
In-Depth Information
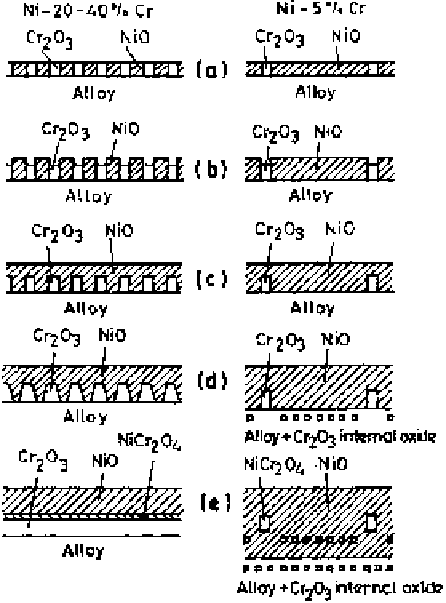
Figure 6.10
Schematic representation of the transient oxidation of Ni-Cr alloys [21].
It should be noted that some NiCr
2
O
4
is probably formed directly immediately and by
reaction between NiO and Cr
2
O
3
, but is omitted from the diagrams during the early stages
for simplicity's sake.
slightly miscible; rather they tend to react in forming the compound NiCr
2
O
4
.
Because Cr is more reactive, it is preferentially oxidized and produces the thermo-
dynamically favored Cr
2
O
3
doped with Ni
2
ions on the alloy surface. Neverthe-
less, some NiO doped with Cr
3
ions and NiCr
2
O
4
may also form directly during
the transient stage, but the compound NiCr
2
O
4
can also form by the reaction
between NiO and Cr
2
O
3
.
On exposure of the alloys to an oxidizing atmosphere at high temperature,
small NiO, Cr
2
O
3
, and NiCr
2
O
4
nuclei develop rapidly on and in the alloy surface
from the amorphous skin or prior air-formed skin and grow quickly until they
impinge on one another. The nuclei of NiCr
2
O
4
are omitted in the figure during