Environmental Engineering Reference
In-Depth Information
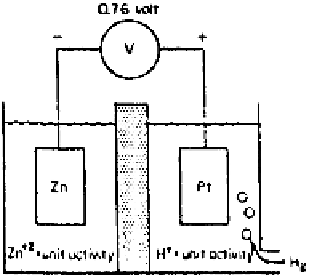
Figure 2.8
Cell containing reversible zinc and standard hydrogen electrode.
It must, however, be emphasized that the actual absolute value for E
H
/H
2
is not
zero.
A standard hydrogen electrode is coupled with another electrode kept in its
standard state, i.e., a metal in equilibrium with its ions at unit activity at 25
C,
and the measured potential is termed the standard single-electrode potential or
standard half-cell potential for the reaction occurring at that electrode. For exam-
ple, as in Fig. 2.8, the single-electrode potential of the zinc electrode, E
Zn
2
/Zn
,
corresponding to the equilibrium
°
Zn
2
2e
i Zn
(2.20)
is
0.76 V. This value is often written as
E
Zn
2
/Zn
0.76 V(SHE)
where SHE refers to standard hydrogen electrode. Similarly, the standard single-
electrode potential for the copper electrode corresponding to the equilibrium
Cu
2
2e
i Cu
(2.21)
is
0.34 V (SHE).
2.2.4 EMF series and Corrosion Prediction
A listing of the standard single-electrode potentials constitutes the electromotive
force series, or the emf series (Table 2.1). The potentials are referred to as redox
potentials as well, meaning that this potential is the equilibrium potential for
reduction and oxidation reactions. It is to be pointed out that the reactions shown
in the table include reactions of O
2
/H
2
O and O
2
/OH
equilibria that are of im-
mense importance in corrosion processes. It is also important to note that some