Environmental Engineering Reference
In-Depth Information
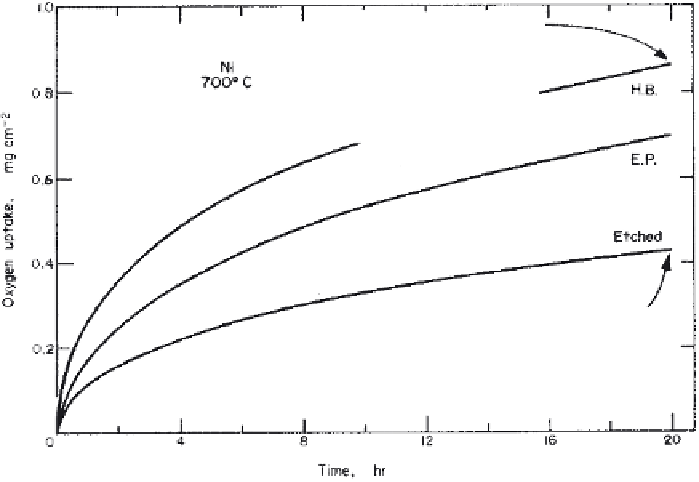
Figure 5.36
Effect of surface pretreatment on the kinetics of oxidation of polycrystal-
line Ni at 700°C and 0.5 torr O
2
pressure. Scanning electron micrographs of the outer
surface of the oxides illustrate a uniform fine-grained oxide on H. B. (''hot-bare'') Ni
compared with a marked variation in oxide thickness with substrate orientation on ''fur-
nace-raised'' ETCHED Ni [Ref. 64].
0.5 torr,
demonstrating the effect of oxidation procedures and surface pretreatments. It
clearly depicts how substrate orientation affects oxide grain size and oxidation
rate. Similar to observations at 973 K, the HB specimen exhibits the fastest oxida-
tion; however, EP Ni is found to oxidize at a slower rate than ETCHED Ni,
contrary to the results at 973 K. After about 40 min exposure, however, an en-
hanced rate of oxidation is observed for EP Ni. At greater oxide thickness,
ETCHED Ni undergoes oxidation at a relatively slower rate consistent with the
results as shown in Fig. 5.36.
On polycrystalline Ni, the kinetic data represent an average oxidation rate of
different substrate orientations. Experimentation on a single crystal hemisphere
shows that orientations close to (112) after EP oxidizes at a much reduced rate,
where only
Figure 5.37 shows the early stages of Ni oxidation at 873 K in
P
O
2
10 nm of oxide film is formed in 1 h. Reflection electron diffraction
has established the oxide to be single crystal [(111) antiparallel NiO on (111)
steps of the (112) macrosurfaces], and its low growth rate is a consequence of the