Environmental Engineering Reference
In-Depth Information
come by using a reference electrode whose potential has arbitrarily been assigned
a zero value. A standard hydrogen electrode is such a reference electrode. When
this is coupled with another electrode, a cell is formed whose potential eventually
corresponds to the single-electrode potential of the second electrode because the
reference electrode has a zero potential.
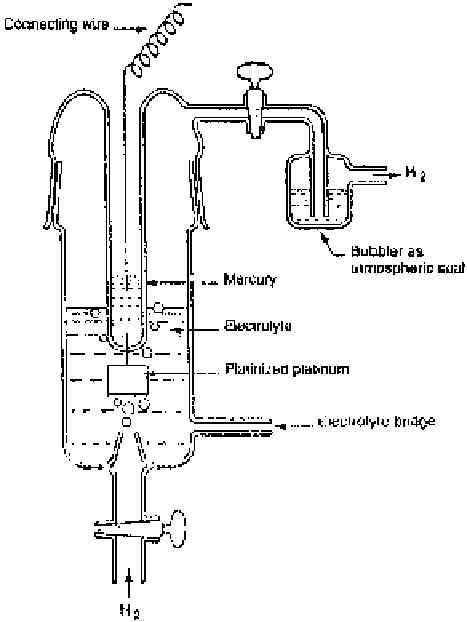
The standard hydrogen electrode comprises a platinized platinum electrode
immersed in a solution having unit activity of hydrogen ions through which hy-
drogen gas is bubbled under one atmospheric pressure (Fig. 2.7). The entire setup
is maintained at 25
C.
The potential of this electrode is expressed by E
0
H
/H
2
°
0 and this corresponds
to the equilibrium of the reaction,
2H
2e
i H
2
(2.22)
Figure 2.7
Schematic representation of standard hydrogen electrode (SHE).