Environmental Engineering Reference
In-Depth Information
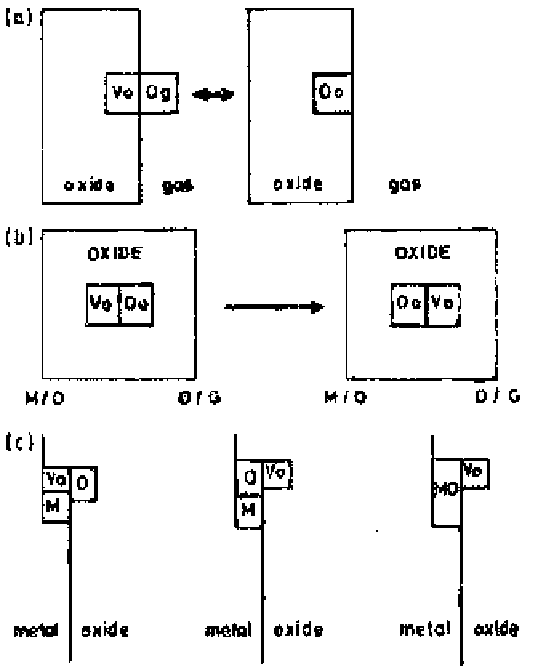
Figure 5.25
Volume changes associated with oxide growth by anion diffusion [Ref.
51].
For the particular case of Zr/ZrO
2
considered above,
φ
1.56 so that
∆Ω
A
0.56
Ω
M
.
In such calculations for oxidation of zircalloy-2, Evans [51] estimated
∆Ω
to
be positive but about two-thirds the value of
φ
. The rate of reaction was thus
expected to be less in the stressed oxide, i.e.,
was negative in Eq. 5.109.
The negative stress in the oxide is known to increase with oxide thickness which
means that retardation in the rate will also increase with thickness. As a conse-
quence, the kinetics of reaction will be subparabolic and thus in accord, at least
qualitatively, with common observations on this alloy.
It has been further pointed out that there exists the possibility for the stress,
either originating from the growth process or deriving from external constraints,
σ
H
∆Ω