Environmental Engineering Reference
In-Depth Information
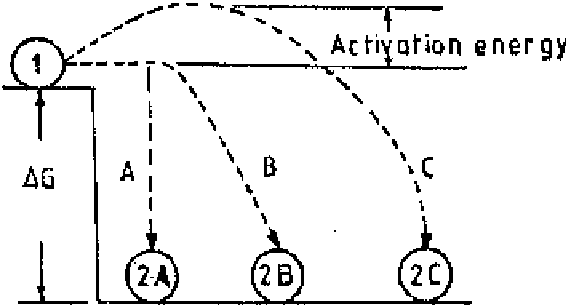
tion. The change in free energy is a state function and is independent of the
reaction path, but the reaction rate is dependent on the path followed. This is
exemplified in Fig. 2.5.
Position 1 is at a higher free energy state than position 2, and the difference
in free energy (
G
) has a negative value when the transformation takes place
from position 1 to position 2. The negative value is indicative of a spontaneous
direction of transformation and it is the same whether the path followed is A, B,
or C. The path B or C is visibly longer and the transformation or reaction rate
along these paths has to be slower than along the path A. The path C has a hump.
The reaction will not proceed from the trough position across the hump to position
2C unless some additional energy, called
activation energy
, is provided. Thus a
negative free energy change is neither a guarantee for the reaction nor an indica-
tion for the rate at which it may proceed. On the other hand, a positive value of
free energy change, like that for the transformation or reaction from position 2
to position 1, indicates that it is to be achieved only with the supply of additional
energy and is not a spontaneous direction for reaction.
The free energy change accompanying an electrochemical reaction, like a cor-
rosion reaction, can be calculated as follows:
∆
∆
G
nFE
(2.16)
where
∆
G
is the free energy change, in joules,
n
is the number of electrons involved in the reaction,
F
is the Faraday constant, in coulombs,
E
is the cell potential, in volts.
Figure 2.5
Effect of reaction path on reaction rate.