Environmental Engineering Reference
In-Depth Information
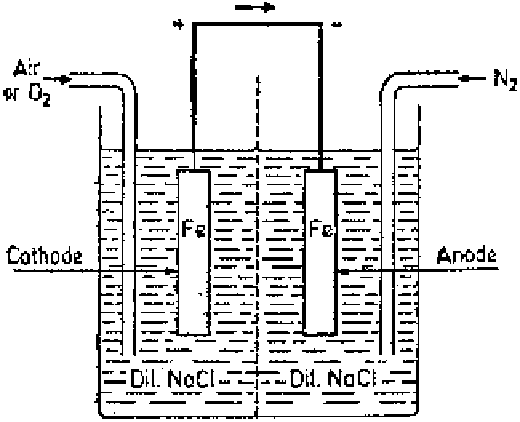
Figure 2.4
Differential aeration cell.
2.1.4 Homogeneous Theory of Corrosion
Corrosion essentially involves charge transfer reactions at the metal-electrolyte
interface and these have been termed as anodic and cathodic reactions depending
on whether the reaction releases electrons or consumes electrons. Both reactions
proceed simultaneously and at the same rate, so that there is no charge accumula-
tion in the corroding metal. In the local cell theory of corrosion, anodic and
cathodic reactions have been visualized to occur at distinctively different sites,
called anode and cathode.
As discussed earlier, heterogeneities of one type or another tend to fix up the
anodic and cathodic sites on a metal surface. Nevertheless, corrosion will proceed
even if no such heterogeneity is present, as in the case of ultrapure metals. This
is explained as follows: The necessary and sufficient condition for corrosion is the
metal dissolution reaction and some electronation (reduction) reactions proceed
simultaneously at the metal-environment interface. For these two processes to
take place simultaneously, it is necessary and sufficient that the potential differ-
ence across the interface be more positive than the equilibrium potential of the
reaction
n
e
(2.6)
and more negative than the equilibrium potential of the electronation reaction,
say,
M
M
n