Environmental Engineering Reference
In-Depth Information
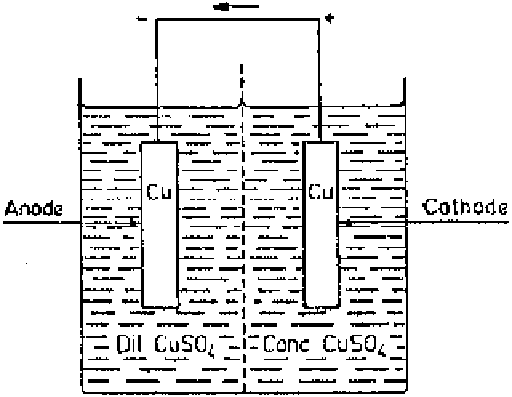
Figure 2.3
Salt concentration cell.
illustrated in Fig. 2.4. The electrode in contact with the less aerated or oxygenated
solution will act as anode.
Differential aeration cell formation is quite common in practice and is very
important from the viewpoint of practical corrosion damages. A metallic bucket
half-filled with water tends to get corroded just below the water line because of
lower oxygen concentration compared to the area just above it near the water
line. Corrosion damage invariably becomes pronounced underneath a corrosion
product or at crevices where oxygen availability is low. Formation of concentra-
tion cells of both kinds account for the initiation of pits (discussed later in Section
3.4) in stainless steels or in some other metals and alloys exposed to seawater.
Differential Temperature Cells
These cells are formed when electrodes of the same metal, each of which is at
a different temperature, are immersed in an electrolyte of the same initial compo-
sition. Such a situation may arise in practice in components of heat exchangers,
boilers, and similar equipment. Polarity developed on an electrode varies from
system to system. For a copper electrode in copper sulfate solution the electrode
at higher temperature is cathode, but for lead the situation is just the reverse. For
iron immersed in dilute aerated sodium chloride solutions, the hot electrode is
initially anodic to the colder metal, but the polarity may reverse with the progress
of corrosion.