Environmental Engineering Reference
In-Depth Information
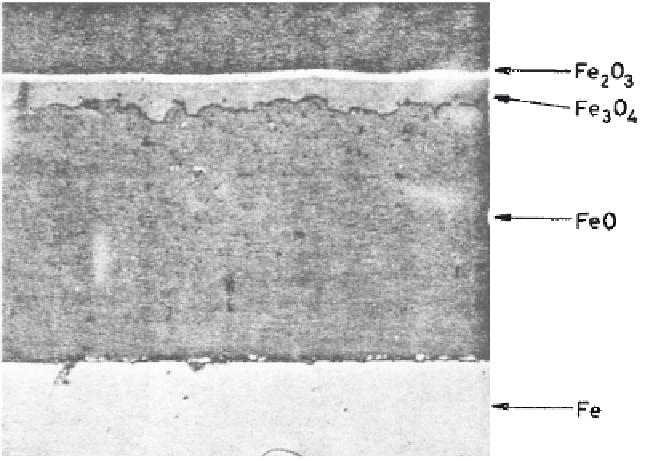
Figure 5.4
Microsection of scale formed on iron in air at 625°C after 24 h [Ref. 2].
3.
Predominant transport processes in space charge boundary layers in the case
of thin tarnishing films, especially at low temperatures.
Besides these, there are two more significant aspects that need due consideration:
1.
Thermodynamic stability of reaction product, and
2.
Crystal structure and morphology of the oxide scale as well as that of the
underlying metal or alloy.
Some of the main aspects of partial processes during metal oxidation are pre-
sented schematically in Fig. 5.6 [4].
If one starts with a theoretically clean metal surface exposed to an oxidant,
the initial step in the interaction is adsorption of the oxidant on the metal surface.
On continued exposure to the reacting environment, the initial nuclei of the reac-
tion product are formed at some preferential sites. These often grow laterally to
produce a continuous film that ultimately covers the entire surface of the metallic
substrate. In certain situations the oxidant may get dissolved in the metal substrate
to an extent determined by the solubility and diffusivity of the oxidant in the
metal at the temperature under consideration. Once the continuous adherent film
is formed, it separates the reactants by creating a physical barrier for which further