Environmental Engineering Reference
In-Depth Information
In pure state,
a
MO
2
1,
a
M
1, and
a
O
2
(
P
O
2
/
P
O
2
)
P
O
2
(since the standard
state of O
2
is unity, i.e.,
P
O
2
1 atm). Therefore
∆
G
T
RT
ln
P
O
2
∆
H
0
T
∆
S
0
(5.5)
From the thermodynamic data available in literature, at any temperature
T
,
equilibrium
P
O
2
values at M/MO
2
and MO
2
/O
2
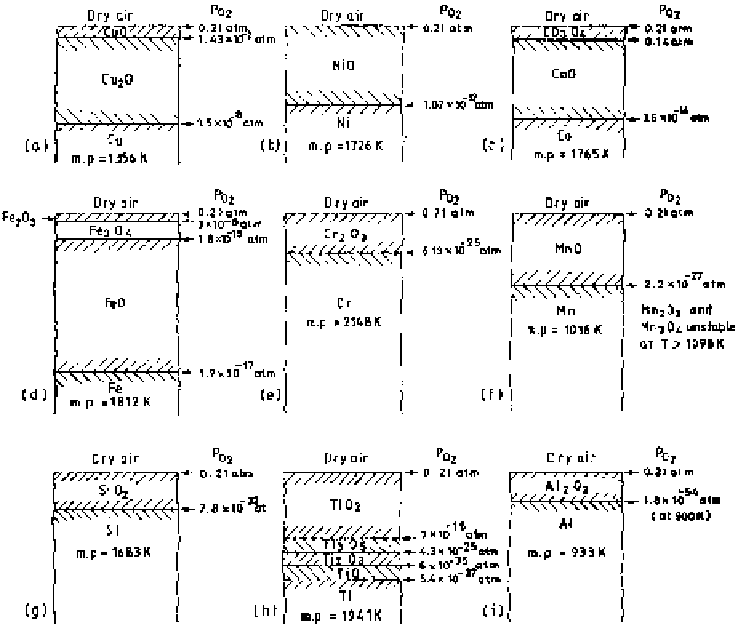
can be estimated. If a metal is
exposed at some elevated temperature in dry air, there may be formation of single
oxide or multilayered oxides. For some common metal-air (dry) systems, assum-
ing compact oxide scale formation, the equilibrium
P
O
2
values developed at vari-
ous interfaces at 1173 K are presented in Fig. 5.2a-i.
Another relevant graphical representation of metal-oxidant equilibria, which
Figure 5.2
Schematic presentation of single-layered and multilayered oxide scale for-
mation in dry air at 1173 K for different metal-oxygen systems showing equilibrium
values of P
O
2
at different reaction interfaces (assuming compactness of the scales).