Environmental Engineering Reference
In-Depth Information
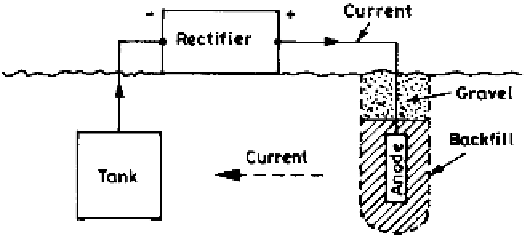
Figure 4.11
Schematic representation of an impressed current cathodic protection
system.
not be consumed, but in practice they are consumed slowly and need replacement.
Platinized titanium has indefinite life as an auxiliary anode and is being increas-
ingly used in the protection of marine structures.
It is important to remember that all buried parts of the ground bed assembly
connected to the positive terminal of the power source may discharge current if
they come in contact with the soil and corrode. The cable from the rectifier to
the anodes or the cables interconnecting the anodes in the ground bed may eventu-
ally snap in that process, disrupting the electrical contact of the installation.
Proper insulation of these parts is, therefore, of utmost importance. By contrast,
all buried wires connecting to the sacrificial anodes collect current from the envi-
ronment and are protected.
Protecting currents are usually determined empirically. The current require-
ment is low in static environments but increases under flowing conditions of the
environment. It becomes enormously high in aggressive media such as for steel
in hot sulfuric acid, and cathodic protection is not economically viable in such
cases. For buried steel structures in soils, a potential difference of
0.85 V with
respect to a copper-copper sulfate reference electrode is held as the criterion of
protection. Current is adjusted to maintain this potential difference. For the pro-
tection of lead-seathed cables, this value is
0.70 V, and for aluminum between
the limits of
1.20 Volt. The upper limit is specified for aluminum
to avoid alkalinity buildup at the cathode (''cathodic corrosion''). As in the case
of a sacrificial anode system, insulating coatings must be used on the structures
to be protected in order to reduce the total current requirement.
The effectiveness of protection is monitored through potential measurement.
Test coupons made of the same metal as that in the protected structures are often
exposed in electrical contact with the protected structure. Any loss in weight will
indicate the inadequacy of protection. Cotton soaked in potassium ferricyanide
is sometimes placed in contact with the protected structure. It turns blue with
1.00 and