Environmental Engineering Reference
In-Depth Information
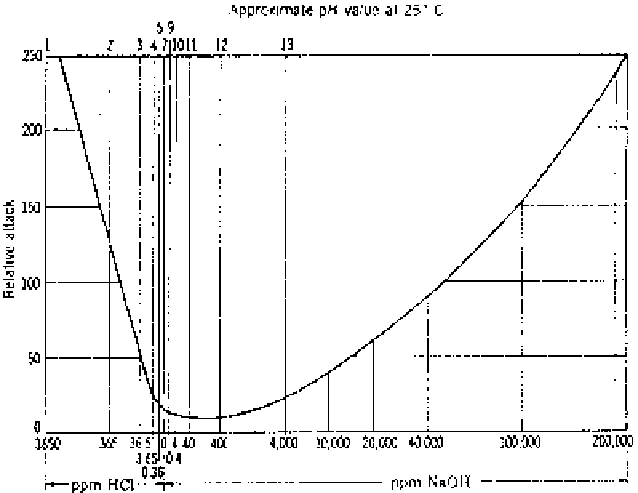
Figure 4.5
Corrosion of iron by water at various pH values.
inated acid is also highly corrosive toward nickel-molybdenum alloys, which
possess excellent corrosion resistance in pure hydrochloric acid. Removal of such
oxidizers naturally reduces corrosion.
Moisture is an aggressive corrosive constituent in the atmosphere. Lowering
of relative humidity of air by increasing the temperature by 6-7
C above ambient
in storage areas brings down the rate of corrosion. Removal of moisture by use
of silica gel in small closed spaces reduces corrosion.
Oxygen is a strong cathodic depolarizer and the presence of dissolved oxygen
is the cause for corrosion of steel in waters above pH 6.0. Neutral water contains
about 8 ppm of dissolved oxygen at 20
°
C, whereas only 0.1 ppm of oxygen is
required to increase corrosion rates in a dynamic system, e.g., in boilers. The
removal of oxygen from feedwater is therefore a necessary step in boiler opera-
tions. In high-pressure boilers, the maximum allowable oxygen concentration in
feedwater is 0.005 ppm.
Removal of dissolved oxygen from water is accomplished either by
deaeration
or by
deactivation
. Deaeration is the process of distilling off of the oxygen in
suitable equipment and deactivation refers to the removal of oxygen by chemical
reaction.
Deaeration is carried out by spraying water countercurrent to steam, by inert
°