Environmental Engineering Reference
In-Depth Information
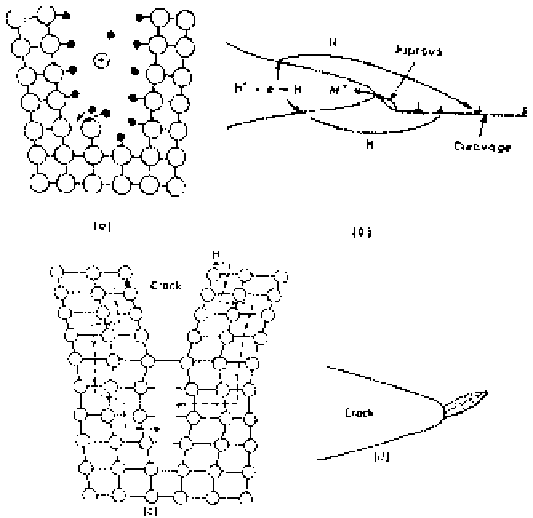
Figure 3.49
Hydrogen-assisted cracking mechanisms. (a) Crack tip adsorption and
bond rupture. (b) Embrittlement due to anchoring of dislocations by adsorbed hydrogen.
(c) Decohesion by hydrogen influx to dilated lattice. (d) Crack extension due to brittle
hydride phase formation.
SCC in these alloys. Again, all of the environments and conditions in which SCC
is encountered do not produce hydrogen, and some of them produce surface films
that constitute an effective barrier to hydrogen entry. These restrict the universal
applicability of hydrogen-induced cracking as an SCC mechanism.
The adsorption-induced cleavage
mechanism, or the stress-sorption cracking
mechanism [32], is based on the hypothesis that adsorption of environmental
species lowers the interatomic bond strength and the stress required for cleavage
fracture—an idea similar to the decohesion model of hydrogen-induced cracking.
Adsorption is assumed to be potential-dependent, which accounts for the stoppage
of SCC by cathodic polarization below a critical potential. The specificity of the
species for inducing SCC in a particular alloy can also be conveniently explained
in terms of preferential adsorption. However, this model does not explain how
the crack maintains an atomically sharp tip in a normally ductile material, and
it also fails to explain the discontinuous nature of crack propagation.
The tarnish rupture mechanism
was originally proposed to explain discontinu-