Environmental Engineering Reference
In-Depth Information
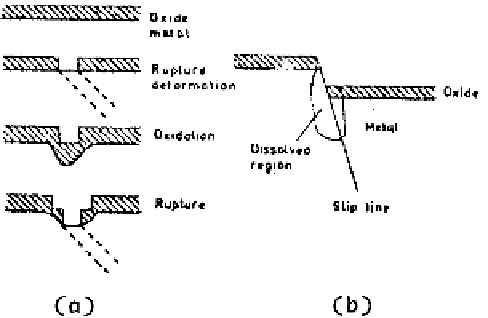
Figure 3.47
Strain-generated active path mechanisms. (a) Film rupture model. (b)
Slip-step dissolution model.
a change in the morphology of corrosion damage from tunnels to thin, flat slots,
as shown in Fig. 3.48b. The width of the corrosion slots has been shown to
approach atomic dimensions and a close correspondence of matching surfaces is
expected.
Cleavage Mechanisms.
Several mechanisms have been postulated to explain
the cleavage-type cracking encountered in SCC. These are hydrogen-assisted
cracking, turnish rupture, film-induced cleavage, adsorption-induced cleavage,
and atomic surface mobility mechanisms.
Hydrogen-assisted cracking mechanism
is very often described by the surface
energy lowering model [30] in which atomically dissolved hydrogen acts to
weaken the interatomic bonds in the plain-strain region of the crack tip (Fig.
3.49) by lowering the surface energy
γ
s
in the Griffith equation:
σ
c
(2
E
γ
s
/
π
c
)
1/2
(3.7)
where
σ
c
is the fracture stress necessary to cause the propagation of an elliptical
crack of length 2
c
and
E
is Young's modulus. Blocked glide planes have been
considered to provide the initial Griffith cracks in steels. The decohesion may
also be caused by hydrogen influx to the dilated lattice [31]. It has also been
suggested that hydrogen decreases the stacking-fault energy to induce coplanar
deformation at the tip. Embrittlement would then result from Lomer-Cottrell
supersessile dislocations on the intersecting slip planes. The embrittlement has
also been ascribed to the stress-assisted formation of brittle hydride phase ahead
of the crack tip which facilitates crack growth by cleavage, with cracks arresting
at the boundary where the relatively tough matrix is encountered. Another particle