Environmental Engineering Reference
In-Depth Information
Carbamate pesticides undergo hydrolysis, aliphatic side-chain oxidation, thioether oxi-
dation, methylation, N-dealkylation, and rearrangement reactions when exposed to light
(Tamini et al. 2006). In the presence of UV light, carbamate pesticides in water undergo
cleavage of the ester bond, resulting in the production of the phenol or heterocyclic enol of
carbamate ester. The hydrolysis products produced further undergo photodecomposition
to form a number of products.
Methomyl, oxamyl, and oxime derivatives used as insecticides, on photolysis, form a
number of products.
H
3
C
O
N
C
CH
3
O
N
C
H
S
CH
3
CH
2
O
CH
3
HO
N
C
CH
3
HO
C
N
O
N
C
H
S
CH
3
S
CH
3
H
O
N
CH
3
C
H
O
C
N
S
CH
3
C
CH
3
N
O
O
O
CH
2
C
C
C
CH
3
OH
CH
3
NH
2
OH
HO
O
O
O
C
C
C
H
CO
2
OH
OH
HO











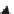















Search WWH ::

Custom Search