Environmental Engineering Reference
In-Depth Information
Regenerated GAC
Virgin GAC
2.5
2.0
1.5
1.0
0.5
0.0
0
5
10
15
V
/dm
3
g
-1
20
25
30
35
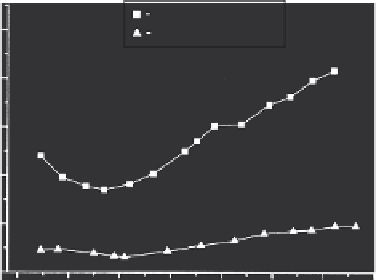
FIGURE 9.1
Cumulative breakthrough curves for 10 different chlorinated pesticides on virgin and regenerated granular
activated carbon (GAC). Total pesticide concentration in the influent is 20 μg/L. (From Ninković, M. B., Petrović,
R. D., and Laušević, M. D. 2010. Removal of organochlorine pesticides from water using virgin and regenerated
granular activated carbon.
J. Serb. Chem. Soc.
75, 565-573. With permission.)
as coagulation, flocculation, or sedimentation (Pehkonen and Zhang 2002). Therefore,
more efficient processes, such as activated carbon adsorption or ozonization, are needed
to remove pesticides during drinking water production (Ormad et al. 2008).
Activated carbon adsorption process seems to be the most feasible process for removing
pesticides. It has adsorption affinity for contaminants that are hydrophobic. Therefore, a
majority of pesticides that exhibit low solubility in water are efficiently removed on fixed-
bed adsorbers with carbon, used in water purification plants. Many studies concerning
the adsorption capacities of selected types of pesticides on commercially activated carbons
have been published (Schreiber et al. 2005; Daneshvar et al. 2007). The adsorption capacity
of activated carbon to remove pesticides is affected by competition from other contami-
nants or natural organic matter that blocks the active seats (Pelaekani and Snoeyink 2000).
After saturation, used activated carbon is regenerated and reused in order to reduce the
cost of water production and minimize waste. The adsorption capacity of regenerated acti-
vated carbon is considerably lower compared to the virgin activated carbon (Figure 9.1). In
addition, rinsing of the pesticides after the saturation point is a far more efficient process
on regenerated carbon (Ninković et al. 2010).
Consequently, there is a growing interest in developing new carbon adsorbents,
which are more effective for the removal of micropollutants, including pesticides
(Baćić-Vukčević et al. 2006). Hence, activated carbon fibers (Martin-Gullon and Font
2001), carbon cloth (Vasiljević et al. 2004b, 2006; Ayranci and Hoda 2004), and other
alternative carbon materials (Vukčević et al. 2008) with higher adsorption capacity
and kinetics have been investigated as a possible replacement for activated carbon
in water purification plants. Among all the recently developed nanoadsorbents for
drinking water purification, the chemistry of noble metal nanoparticles is truly unique.
Synergistic effects could be driven by the use of silver nanoparticles loaded on activated
carbon substrate. Both components work synergistically to remove a variety of pesti-
cides from drinking water (Anshup 2009).
Ozone is primarily used as a disinfectant. However, as a powerful oxidant, ozone has
many uses in water treatment, including the oxidation of pesticide residues. The intensive
Search WWH ::

Custom Search