Environmental Engineering Reference
In-Depth Information
H
3
C
S
N
C
S
S
CH
3
H
3
C
C
N
SO
2-
, CO
2
S
CH
3
(CH
3
)
2
NH
R-S
SO
2-
h
+
OH,
OOH,
OH, OOH,
OH,
OOH,
h
+
h
+
h
+
OH, OOH,
CO
2
H
3
C
O
N C
CH
3
(CH
3
)NH
2
S
S
H
3
C
C N
CH
3
S
OH, OOH, h
+
SO
2-
OH, OOH, h
+
CO
2,
NH
+
,
NO
-
O
H
3
C
N
C
H
3
C
S
S
CH
3
C N
O
CH
3
FIGURE 6.14
Proposedphotocatalyticdegradationpathwayofthiram.(Reprintedfrom
Chem. Eng. J.,
148,Kaneco,S.,Li,N.,
Itoh,K.-K.,Katsumata,H.,Suzuki,T.,andOhta,K.,Titaniumdioxide-mediatedsolarphotocatalyticdegrada-
tionofthiraminaqueoussolution:Kineticsandmineralization,50-56,(2009),withpermissionfromElsevier.)
6.5.4.2 Phenylurea Herbicides
Thenitrate-inducedphotodegradationofphenylureaherbicidesinwaterwasdescribedby
Shankaretal.(2008)tooccureficientlyusingnaturalsunlightirradiation.Sharmaetal.
(2008) described photocatalytic degradation and mineralization of pesticides including
thephenylureaherbicideisoproturonoverTiO
2
-supportedmesoporousSBA-15composite
system using sunlight. Silva et al. (2010) described photodegradation of the phenylurea
herbicidediuronandphosphoramideinsecticidefenamiphos,andthephotoproductswere
identiiedbyLC-MS.
6.5.4.3 Phenoxypropionic Herbicides
Photodegradationofmecopropanddichlorpropondry,moist,andamendedsoilsurfaces
exposedtosunlightwasdescribedbyRomeroetal.(1998).Conclusionsproposedbythe
authors(Romeroetal.1998)werethefollowing:
• A slow rate of disappearance of mecoprop and dichlorprop occurs in the three
soilsexposedtosunlightintheabsenceofwater.Nevertheless,indryconditions,
photolysismaydominateoverothertransformationpathways











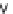


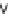



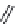












































Search WWH ::

Custom Search