Environmental Engineering Reference
In-Depth Information
*
S
Et
3
N
CH
3
O
*
Cl
P
PSCl
3
+ 2 CH
3
OH
*
CH
3
O
KO
NO
2
S
CH
3
O
CH
3
O
P
NO
2
*
Reflux, 2-3 hrs
CH
3
O
CH
3
Fenitrothion [I]
CH
3
COOH
S
S
S
CH
3
O
CH
3
O
CH
3
O
CH
3
O
CH
3
O
CH
3
O
NO
2
NO
2
P O
NO
2
O
P
P O
[I]
[IV]
[VII]
CH
3
CH
3
COOH
COOH
O
P
O
CH
3
O
CH
3
O
CH
3
O
CH
3
O
HO
NO
2
O
NO
2
P
O
NO
2
HO
NO
2
[VI]
[III]
[II]
[V]
FIGURE 6.13
Synthesis of
14
C-fenitrothion and its main degradation products by photodecomposition. (Reprinted from
Chemosphere,
70, Zayed, S. M. A. D. and Mahdy, F., Decomposition of
14
C-fenitrothion under the inluence of
UVandsunlightundertropicalandsubtropicalconditions,1653-1659,(2008),withpermissionfromElsevier.)
AdetailedUV-Aphotolysismechanismofazinphos-methylwasproposedbyYeasmin
et al. (2009), involving two pathways, the major one leading to benzazimide as a stable
photoproductandtheothertoN-methylanthranilicacidasanintermediateandanilineas
ainalstablephotoproduct.Thisphotolysishasimplicationsforluorescence-basedtrace
analysis of this pesticide, as controlled UV exposure results in signiicant luorescence
enhancementofazinphos-methylinsolutionviatheformationofthehighlyluorescent
intermediateN-methylantranilicacid(Yeasminetal.2009).
Kralj et al. (2007) described applications of bioanalytical techniques in evaluating
advancedoxidationprocessesinpesticidedegradation.ThewidelyacceptedMicrotoxtest
appears to be the most versatile and suficiently rapid and sensitive method. However,
thebioluminescenceof
Vibrio ischeri
usedintheMicrotoxtestisnotalwaysanadequate
indicatorofneurotoxiccompoundsthatcanbeproducedduringadvancedoxidationdeg-
radationprocessesofsomeorganophosphoruspesticides.Kraljetal.(2007)demonstrated
thattheacetyl-cholinesterasebioassay,basedonthermallensspectrometricdetermination
ofenzymaticactivityinalow-injectionanalysissystemisausefultool.Thephototrans-
formationsoftwoorganophosphoruspesticides,parathionandchlorpyrifos,byhydroxyl
radicalsandcarbonateradicalsinaqueoussolutionwerestudiedbyWuandLinden(2010).
AdditionofhydrogenperoxideincreasedtheUVdegradationratesofbothpesticidesand
the data were simulated through kinetic modeling. The second-order rate constants of
parathion and chlorpyrifos with hydroxyl radical were determined to be 9.7±0.5×10
9











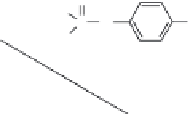



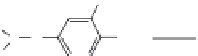

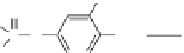






Search WWH ::

Custom Search